Freezing lithium batteries may make them safer and bendable
Columbia Engineers use ice-templating to control electrolyte structure in lithium batteries
Yuan Yang, assistant professor of materials science and engineering at Columbia Engineering, has developed a new method that could lead to lithium batteries that are safer, have longer battery life, and are bendable, providing new possibilities such as flexible smartphones. His new technique uses ice-templating to control the structure of the solid electrolyte for lithium batteries that are used in portable electronics, electric vehicles, and grid-level energy storage.
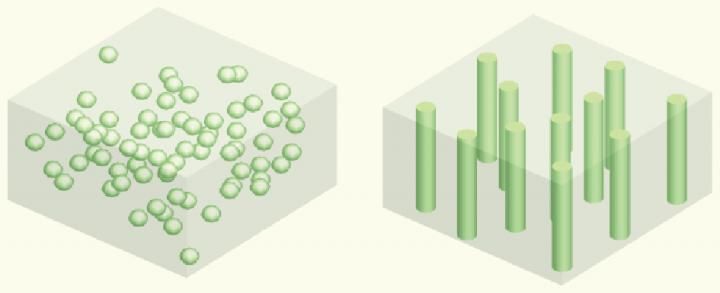
Schematic of vertically aligned and connected ceramic channels for enhancing ionic conduction. In the left figure, ceramic particles are randomly dispersed in the polymer matrix, where ion transport is blocked by the polymer matrix with a low conductivity. In the right one, vertically aligned and connected structure facilitates ion transport, which can be realized by the ice-templating method.
Yuan Yang/Columbia Engineering
Liquid electrolyte is currently used in commercial lithium batteries, and, as everyone is now aware, it is highly flammable, causing safety issues with some laptops and other electronic devices. Yang's team explored the idea of using solid electrolyte as a substitute for the liquid electrolyte to make all-solid-state lithium batteries. They were interested in using ice-templating to fabricate vertically aligned structures of ceramic solid electrolytes, which provide fast lithium ion pathways and are highly conductive. They cooled the aqueous solution with ceramic particles from the bottom and then let ice grow and push away and concentrate the ceramic particles. They then applied a vacuum to transition the solid ice to a gas, leaving a vertically aligned structure. Finally, they combined this ceramic structure with polymer to provide mechanical support and flexibility to the electrolyte.
"In portable electronic devices, as well as electric vehicles, flexible all-solid-state lithium batteries not only solve the safety issues, but they may also increase battery energy density for transportation and storage. And they show great promise in creating bendable devices," says Yang, whose research group is focused on electrochemical energy storage and conversion and thermal energy management.
Researchers in earlier studies used either randomly dispersed ceramic particles in polymer electrolyte or fiber-like ceramic electrolytes that are not vertically aligned. "We thought that if we combined the vertically aligned structure of the ceramic electrolyte with the polymer electrolyte, we would be able to provide a fast highway for lithium ions and thus enhance the conductivity," says Haowei Zhai, Yang's PhD student and the paper's lead author. "We believe this is the first time anyone has used the ice-templating method to make flexible solid electrolyte, which is nonflammable and nontoxic, in lithium batteries. This opens a new approach to optimize ion conduction for next-generation rechargeable batteries."
In addition, the researchers say, this technique could in principle improve the energy density of batteries: By using the solid electrolyte, the lithium battery's negative electrode, currently a graphite layer, could be replaced by lithium metal, and this could improve the battery's specific energy by 60% to 70%. Yang and Zhai plan next to work on optimizing the qualities of the combined electrolyte and assembling the flexible solid electrolyte together with battery electrodes to construct a prototype of a full lithium battery.
"This is a clever idea," says Hailiang Wang, assistant professor of chemistry at Yale University. "The rationally designed structure really helps enhance the performance of composite electrolyte. I think that this is a promising approach."
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.