Progress Toward a New Generation of Rechargeable Batteries
Redox mediator improves performance and lifespan of Li-O2 batteries
Lithium–air batteries have the potential to outstrip conventional lithium-ion batteries by storing significantly more energy at the same weight. However, their high-performance values have thus far remained theoretical, and their lifespan remains too short. A Chinese team has now proposed addition of a soluble catalyst to the electrolyte. It acts as a redox mediator that facilitates charge transport and counteracts passivation of the electrodes.
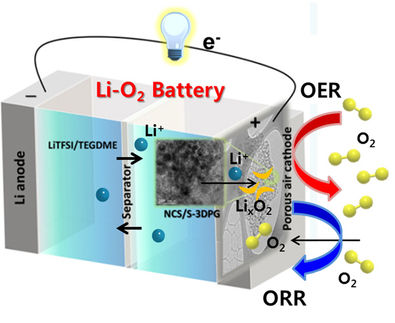
In contrast to lithium-ion batteries, in which lithium ions are “pushed” back and forth between two electrodes, lithium-air batteries (Li-O2) use an anode made of metallic lithium. As the battery is used, positively charged lithium ions dissolve and move over to the porous cathode, which has air flowing through it. Oxygen is oxidized and bound into lithium peroxide (Li2O2). Upon charging, the oxygen is released, and the lithium ions are reduced back to metallic lithium, which deposits back onto the anode. Unfortunately, the theoretically high performance of such batteries has not become a reality.
In practice, an effect known as overpotential slows the electrochemical reactions: the formation and decomposition of insoluble Li2O2 are slow and its conductivity is also very low. In addition, the pores of the cathode tend to become clogged, and the high potential required for the formation of oxygen decomposes the electrolyte and promotes undesirable side reactions. This causes the batteries to lose the majority of their performance after only a few charge/discharge cycles.
A team led by Zhong-Shuai Wu from the Dalian Institute of Chemical Physics of CAS, collaborating with Xiangkun Ma from the Dalian Maritime University, has now proposed the addition of a novel imidazole iodide salt (1,3-dimethylimidazolium iodide, DMII) to act as a catalyst and redox mediator to enhance the performance and lifespan.
The iodide ions (I−) in the salt can easily react to form I3− and then back again (redox pair). In this process, they transfer electrons to oxygen (discharge) and take them back up (charge). This facilitated charge transport accelerates the reactions, reduces the overpotential of the cathode, and increases the discharge capacity of the electrochemical cell. The DMI+ ions from the salt contain a ring made from three carbon and two nitrogen atoms. This ring has freely mobile electrons and can “capture” lithium ions during discharge and effectively transfer them to the oxygen at the cathode. In addition, the DMI+ ions form an ultrathin but highly stable interface film on the anode, which prevents direct contact between the electrolyte and the lithium surface, minimizing the decomposition of the electrolyte and preventing side reactions. This stabilizes the anode and increases the lifespan of the battery.
The electrochemical test cells produced by the team were highly promising, demonstrating a very low overpotential (0.52 V), high cycle stability over 960 hours, and highly reversible formation/decomposition of Li2O2 with no side reactions.
Original publication
Jing Liu, Yuejiao Li, Yajun Ding, Lisha Wu, Jieqiong Qin, Tongle Chen, Caixia Meng, Feng Zhou, Xiangkun Ma, Zhong‐Shuai Wu; "A Bifunctional Imidazolyl Iodide Mediator of Electrolyte Boosts Cathode Kinetics and Anode Stability Towards Low Overpotential and Long‐Life Li‐O2 Batteries"; Angewandte Chemie International Edition, 2025-1-14
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.