New materials could turn water into the fuel of the future
Researchers at Caltech and Lawrence Berkeley National Laboratory (Berkeley Lab) have--in just two years--nearly doubled the number of materials known to have potential for use in solar fuels.

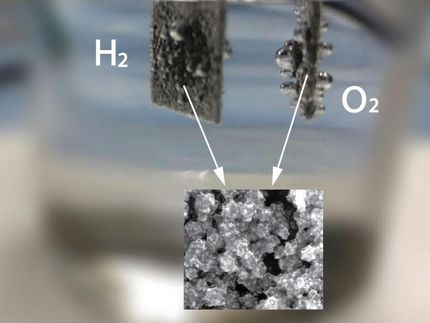
New materials are created through deposition onto disks, which are then tested to determine their properties.
Caltech
They did so by developing a process that promises to speed the discovery of commercially viable solar fuels that could replace coal, oil, and other fossil fuels.
Solar fuels, a dream of clean-energy research, are created using only sunlight, water, and carbon dioxide (CO2). Researchers are exploring a range of target fuels, from hydrogen gas to liquid hydrocarbons, and producing any of these fuels involves splitting water.
Each water molecule is comprised of an oxygen atom and two hydrogen atoms. The hydrogen atoms are extracted, and then can be reunited to create highly flammable hydrogen gas or combined with CO2 to create hydrocarbon fuels, creating a plentiful and renewable energy source. The problem, however, is that water molecules do not simply break down when sunlight shines on them--if they did, the oceans would not cover most of the planet. They need a little help from a solar-powered catalyst.
To create practical solar fuels, scientists have been trying to develop low-cost and efficient materials, known as photoanodes, that are capable of splitting water using visible light as an energy source. Over the past four decades, researchers identified only 16 of these photoanode materials. Now, using a new high-throughput method of identifying new materials, a team of researchers led by Caltech's John Gregoire and Berkeley Lab's Jeffrey Neaton and Qimin Yan have found 12 promising new photoanodes.
The new method was developed through a partnership between the Joint Center for Artificial Photosynthesis (JCAP) at Caltech, and Berkeley Lab's Materials Project, using resources at the Molecular Foundry and the National Energy Research Scientific Computing Center (NERSC).
"This integration of theory and experiment is a blueprint for conducting research in an increasingly interdisciplinary world," says Gregoire, JCAP thrust coordinator for Photoelectrocatalysis and leader of the High Throughput Experimentation group. "It's exciting to find 12 new potential photoanodes for making solar fuels, but even more so to have a new materials discovery pipeline going forward."
"What is particularly significant about this study, which combines experiment and theory, is that in addition to identifying several new compounds for solar fuel applications, we were also able to learn something new about the underlying electronic structure of the materials themselves," says Neaton, the director of the Molecular Foundry.
Previous materials discovery processes relied on cumbersome testing of individual compounds to assess their potential for use in specific applications. In the new process, Gregoire and his colleagues combined computational and experimental approaches by first mining a materials database for potentially useful compounds, screening it based on the properties of the materials, and then rapidly testing the most promising candidates using high-throughput experimentation.
In the work they explored 174 metal vanadates--compounds containing the elements vanadium and oxygen along with one other element from the periodic table.
The research, Gregoire says, reveals how different choices for this third element can produce materials with different properties, and reveals how to "tune" those properties to make a better photoanode.
"The key advance made by the team was to combine the best capabilities enabled by theory and supercomputers with novel high throughput experiments to generate scientific knowledge at an unprecedented rate," Gregoire says.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.