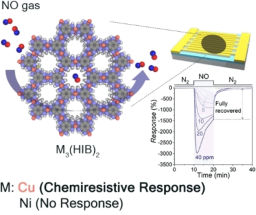
New Technique Developed for Effective Dye Removal and Low-Cost Water Purification
Chinese scientists developed a novel and versatile approach for synthesizing silver-based hybrid nano-particles using laser-induced fabrication, demonstrating the nano-composite’s potential as high-performance adsorbent for dye removal and low-cost water purification
Organic compounds in wastewater, such as dyes and pigments in industry effluents, are toxic or have lethal effect on aquatic living and humans. Increasing evidence has shown that the organic contaminants discharged from electroplating, textile production, cosmetics, pharmaceuticals are the main reasons for the higher morbidity rates of kidney, liver, and bladder cancers, etc. Organic contaminants, especially methyl blue and methyl orange, are stable to light, heat or oxidizing agents and very difficult to remove by conventional chemical or biological wastewater treatment techniques. Recently scientists have developed some new strategies with good dye-removal performance; however, a subsequent adsorbent purification procedure is unavoidable after water treatment, which are often complicated and not suitable for practical water treatment.
Now, using laser-induced fabrication technique, a team of Chinese researchers from Shandong University , China, have developed a novel dye adsorbent. Hybrid nano-particles of silver and silver sulfide (Ag2S@Ag hybrid nano-particles) have demonstrated the nanomaterial’s superior adsorption performance for removing methyl blue and methyl orange from wastewater. More importantly, the new adsorbents can be removed directly from solutions by filters without adsorbent purification procedures, as the silver-based hybrid nano-particles will be agglomerated and deposited on the bottom after adsorbing dyes, providing a green, simple, rapid and low-cost solution for water purification.
“Without using any expensive chemical reagents or facilities, the laser-induced fabrication method is a low-cost, rapid, simple and versatile route for fabricating Ag2S@Ag hybrid nano-crystals,” said Ming Chen, the primary author and an associate professor of School of Physics and State Key Laboratory of Crystal Materials at Shandong University, China. “After adsorbing dyes such as methyl blue and methyl orange, the agglomerated and deposited adsorbents can be easily removed from solutions by filters, which are very beneficial to the practical wastewater treatment plants.”
Chen’s team used a technique called laser ablation to fabricate silver-based hybrid nano-crystals in liquid, which is a process of removing materials from a solid or liquid surface by irradiating it with a laser beam. In a typical experiment, the researchers placed a well-polished silver metal on the bottom of a rotating glass dish filled with thioacetamide solution as a sulfur source for fabricating silver sulfide. The silver surface was then focused by a laser beam, and in a short time, rapid boiling and vaporization of silver element occurred, resulting in explosive silver plasma with ultrahigh temperature (about thousands Celsius) on the irradiated spot. The initial process in crystal formation, or the nucleation of silver and sulfide, took place in the early stage of rapid condensation of the silver plasma, and sharply terminated in a few microseconds due to expiration of the laser pulse and exhaustive expansion of the silver vapor.
“During the fabrication of Ag2S@Ag nanoparticles, the non-equilibrium condition created by subsequent laser ablation leads to various defects, resulting in the presence of abundant disorderly-arranged silver species on the surface of silver sulfide nano-particles and forming a silver shell,” Chen explained. “After laser fabrication, the rapid quenching process enhanced the disorder degree of silver species, making more and more silver atoms at a highly excited state.”
The team’s early study showed that the electron distribution of the highly excited silver species can be influenced by Ag2S@Ag nanoparticles, resulting in “polarized” silver species, or silver species with positive charges as dye adsorption sites. Since dye molecules such as methyl blue and methyl orange have negatively charged functional groups, due to strong electrostatic force between positive charges and negative charges, the enhanced adsorption sites on the hybrid material’s surface will stick more dye molecules, leading to the material’s enhanced capability of removing dyes.
“Our experimental results showed that over 99 percent methyl blue molecules in wastewater were adsorbed by Ag2S@Ag nano-material in five minutes,” Chen said. “The ultraviolet-visible absorption spectra of the solution also clearly showed that after absorbing the dye molecules, the Ag2S@Ag nano-material became agglomerated together into numerous large sized clusters, then deposited on the bottom of the solution. Meanwhile, the aggregation and deposition lead a significant change in the solution color from blue to colorless.”
Chen estimated that after methyl blue adsorption reaction, over 99 percent of Ag2S@Ag nano-material can be removed from solution by filters.
“Compared with conventional adsorbent purification processes, such as centrifugal process or using external magnetic fields, which are complicated and not suitable for practical water treatment, the filter process is simple, rapid and cost-efficient,” Chen said. “Our novel method skips the adsorbent purification process, which promotes the application of Ag2S@Ag nano-particles as advanced adsorbent materials for practical wastewater treatment in the future.”
Since the dye molecules are not easily desorbed from the agglomerated adsorbents by standard heat treatment, the researchers’ next step is to study the desorption method of dye molecules from the Ag2S@Ag adsorbents to realize material recycling in future.












![[Fe]-hydrogenase catalysis visualized using para-hydrogen-enhanced nuclear magnetic resonance spectroscopy](https://img.chemie.de/Portal/News/675fd46b9b54f_sBuG8s4sS.png?tr=w-712,h-534,cm-extract,x-0,y-16:n-xl)