Small is different
Sub-nanometer catalysts show substantially different behavior than projected
In the production of margarine millions of tons of unsaturated fatty acids are converted from vegetable oils using hydrogen. While searching for improved catalysts for these so-called hydrogenation reactions, a German-American research team made a discovery that puts a 50-year old rule in question: In catalytic particles comprising only a few atoms, shape and size influence reactivity much more strongly then previously thought.
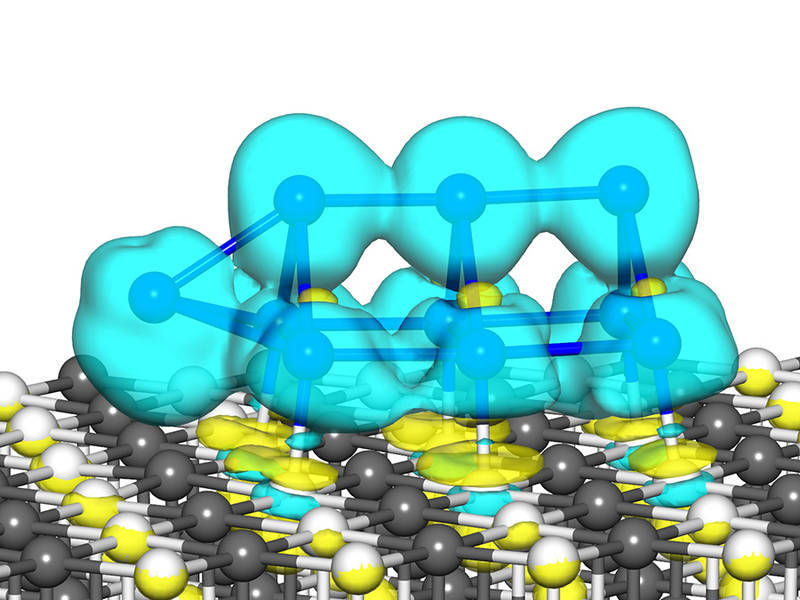
Calculated structure of a Pt10 cluster on a magnesium oxide surface. Light blue represents the excess charge isosurface of the cluster, Pt (blue), Mg (grey), O (white)
U. Landman, B. Yoon / Georgia Tech

Sample holder with three single crystals on which the catalyst particles are deposited
Andreas Heddergott / TUM

Millions of tons of margarine are produced every year by converting unsaturated fatty acids from vegetable oils using hydrogen. While the hydrogenation of vegetable oils is accomplished using cost-effective nickel catalysts, many other reactions require expensive platinum.
Since the hydrogenation reaction takes place only on the surface while the inner atoms play no role at all, industry is developing ever smaller catalytic particles. The smallest of them comprise barely more than 100 atoms. In even smaller particles, however, quantum-mechanical effects take over and conventional models fail to predict the properties of the platinum particles.
A team of researchers at the Technical University of Munich (TUM) and the Georgia Institute of Technology in Atlanta (Georgia) has now investigated these effects with a precision down to the atom. They used the platinum catalyzed reaction converting ethene to ethane as a model. Just like the unsaturated fatty acids, ethene has a carbon double bond. And when two hydrogen atoms react with the double bond, ethene it becomes "saturated" ethane.
A model on shaky ground
For over 50 years chemists have divided catalytic reactions into those that are influenced by the structure and size of a catalyst and those in which these factors play no role. "The ethene hydrogenation was considered a typical example of a size-independent reaction. But our assumption was that this differentiation no longer applies to catalyst particles in the sub-nanometer range," says Ulrich Heiz, chair of the Department of Physical Chemistry at TU Munich, and academic director of TUM's Catalyst Research Center (CRC).
To this end, the working group of Professor Ulrich Heiz produced platinum particles with only a small number of atoms. "With our facility we can specifically produce platinum clusters having one to 80 platinum atoms," says Andrew S. Crampton, a member of the working group. In the presence of these particles they allowed ethene and hydrogen to react and analyzed the results.
The reactivity depends very strongly on the precise number of atoms: Clusters with less than ten atoms were hardly active, while clusters with at least ten atoms showed a successively increasing reactivity, with a maximum at 13 atoms. These particles have significantly higher reaction rates than a normal platinum surface -- clear proof that the size-independency postulated for this reaction in the past decades is inaccurate.
Theoretical models developed by the American colleagues substantiate the experimental results. They now allow the precise determination of which atoms are responsible for which activity and why. "Such small clusters no longer behave as metal bodies, but rather as molecules: Small is different," says Uzi Landman, professor at the Center for Computational Materials Science of the Georgia Institute of Technology. "The properties depend very clearly on the number of atoms."
A well-tuned ensemble
As in the well-known game of Tangram, the atoms of the small clusters can arrange themselves into different forms, chemists refer to as isomers. Furthermore, in clusters comprising only a few atoms interactions with the substrate atoms play an important role.
Meanwhile, the Munich chemists have also developed various processes for fixating the small platinum clusters onto substrates. "This allows us to prevent the small particles from combining into larger ones," explains Ulrich Heiz. "The surface, in turn, influences the predominantly assumed shape of the clusters. Together with the cluster size, we thus have an instrument for customizing the properties for a specific reaction."
In collaboration with members of TUM's Catalyst Research Center, the researchers are about to develop wet chemical processes in the near future to efficiently produce small platinum clusters with precisely defined numbers of atoms in larger quantities.





















































