Adding a polymer stabilizes collapsing metal-organic frameworks
Metal-organic frameworks (MOFs) are a special class of sponge-like materials with nano-sized pores. The nanopores lead to record-breaking internal surface areas, up to 7800 m2 in a single gram. This feature makes MOFs extremely versatile materials with multiple uses, such as separating petrochemicals and gases, mimicking DNA, hydrogen production and removing heavy metals, fluoride anions, and even gold from water - to name a few.
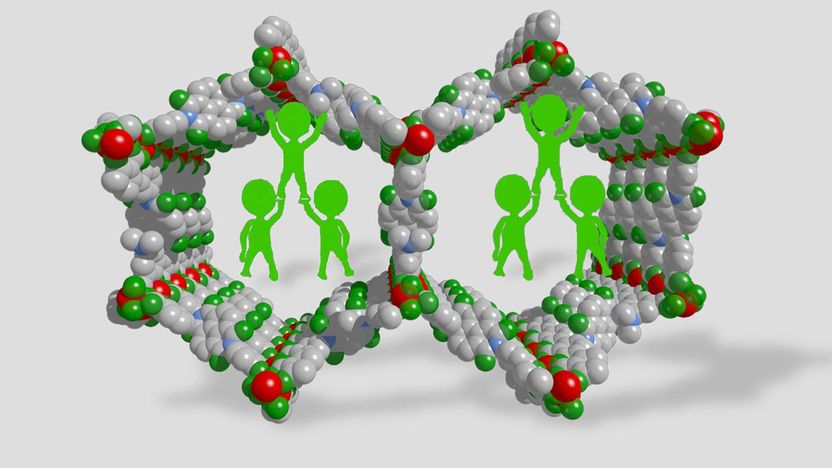
Polymer braces, placed inside large-pore MOFs, help to inhibit the collapse of the framework.
Li Peng (EPFL)
One of the key features is pore size. MOFs - and other porous materials - are classified based on the diameter of their pores: MOFs with pores up to 2 nm in diameter are called "microporous", and anything above that is called "mesoporous". Most MOFs today are microporous, so they are not useful in applications that require them to capture large molecules or catalyze reactions between them - basically, the molecules don't fit the pores.
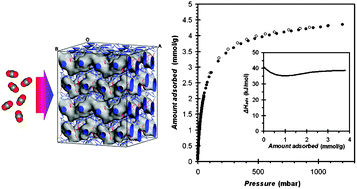
So more recently mesoporous MOFs have come into play, because they show a lot of promise in large-molecule applications. Still, they aren't problem-free: when the pore sizes get into the mesoporous regime - they tend to collapse. Understandably, this reduces the internal surface area of mesoporous MOFs and, with that, their overall usefulness. Since a major focus in the field is finding innovative ways to maximize MOF surface areas and pore sizes, addressing the collapsing problem is top priority.
Now, Dr Li Peng a postdoc at EPFL Valais Wallis has solved the problem by adding small amounts of a polymer into the mesoporous MOFs. Because the polymer pins the MOF pores open, adding it dramatically increased accessible surface areas from 5 to 50 times. The study was led by the research group of Wendy Lee Queen, in collaboration with the labs of Berend Smit and Mohammad Khaja Nazeeruddin at EPFL's Institute of Chemical Sciences and Engineering (ISIC).
After adding the polymer to the MOFs, their high surface areas and crystallinity were maintained even after heating the MOFs at 150°C - temperatures that would previously be unreachable due to pore collapse. This new stability provides access to many more open metal coordination sites, which also increases the reactivity of the MOFs.
In the study, published in the Journal of the American Chemical Society, two PhD students, Sudi Jawahery and Mohamad Moosavi, use molecular simulations to investigate why pores collapse in mesoporous MOFs in the first place, and also propose a mechanism to explain how polymers stabilize their structure on a molecular level.
"We envision that this method for polymer-induced stabilization will allow us to make a number of new mesoporous MOFs that were not before accessible due to collapse," says Queen. "Hence, this work can open up new, exciting applications involving the separation, conversion, or delivery of large molecules."
Original publication
Most read news
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.