Towards mimicking metalloenzymes
Advertisement
Scientists in Germany and the US generate highly oxidised diiron complexes that further our understanding on metalloenzymes in nature.
Oxidised diiron species are used in nature in the active sites of several metalloenzymes, such as methane monooxygenase and ribonucleotide reductase.
Methane monooxygenase catalyses the selective oxidation of methane to methanol and understanding these reaction mechanisms is important in order to develop low-cost methane-to-methanol conversion.
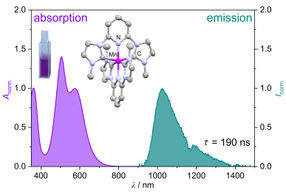
Thorsten Glaser and colleagues from the University of Bielefeld, collaborators from the Max-Planck-Institute for Radiation Chemistry, Mülheim, and co-workers at the Stanford Synchrotron Radiation Laboratory, in California, have oxidised a diferric Fe(III) complex to two Fe(III) radical species. Following decay of these complexes, the identification of a highly reactive Fe(IV) complex ([LFe(IV)=O]) was achieved.
Significantly, the discovery of this Fe(IV) species opens up new pathways to highly oxidised dinuclear complexes which could be applied to biomimetic C-H activation reactions.
‘The future challenges are the enlargement of complexes with more advanced ligands mimicking the second coordination sphere of the enzymes active sites,’ says Glaser.
Studies are already underway in the group, to improve ligand design and stabilise a real dinuclear Fe(IV) complex with reactivities analogous to methane monooxygenase.
Original article: Glaser et. al., Chem. Commun., 2009
Most read news
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.