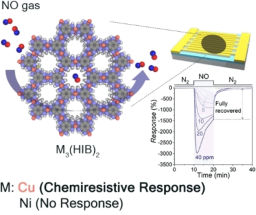
Double dearomatisation
UK scientists use intramolecular coupling between a nucleophilic and an electrophilic heterocycle to synthesise spirocyclic compounds.
Jonathan Clayden and Heloise Brice from the University of Manchester have synthesised spirocyclic compounds via doubly dearomatising intramolecular coupling of a nucleophilic and an electrophilic heterocycle.
The motivation for this work came from the knowledge that simple aromatics are widely available on a large scale, and the products of dearomatisation are valuable reactive intermediates. 'Our starting materials are simple to make' says Clayden, 'while the products have fascinating structural architecture and display a range of further reactivity.'
'In general, aromatic compounds are stable towards dearomatising attack,' explains Clayden. 'In the reaction reported here however, two aromatic rings react with one another and both lose aromaticity as they do so.'
Clayden believes that this ability to construct quite complex structures in one or two steps opens up the possibility of incorporating this type of transformation into a cascade process, and allows diversity to be introduced. 'This type of reaction could be very valuable in the search for inhibitors of enzymes or ligands for receptors,' says Clayden, which is 'the first stage in the discovery of new medicinal agents.'
Original article: Heloise Brice and Jonathan Clayden; Chem. Commun. 2009
Most read news
Organizations
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.







![[Fe]-hydrogenase catalysis visualized using para-hydrogen-enhanced nuclear magnetic resonance spectroscopy](https://img.chemie.de/Portal/News/675fd46b9b54f_sBuG8s4sS.png?tr=w-712,h-534,cm-extract,x-0,y-16:n-xl)