First high-resolution images of bone, tooth and shell formation
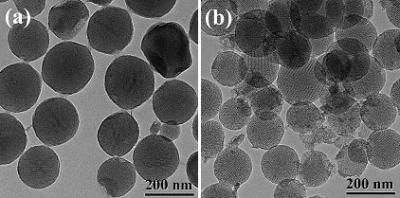
Researchers at Eindhoven University of Technology (TU/e) have for the first time made high-resolution images of the earliest stages of bone formation. They used the world's most advanced electron microscope to make three-dimensional images of the nano-particles that are at the heart of the process. The results provide improved understanding of bone, tooth and shell formation. For industrial applications, they promise better materials and processes based on nature itself. Led by dr. Nico Sommerdijk, the researchers imaged small clusters with a cross-section of 0.7 nanometer in a solution of calcium carbonate (the basic material of which shells are made). They showed for the first time that these clusters, each consisting of only about ten ions, are the beginning of the growth process through which the crystalline biomineral is ultimately formed. To do this they used the very high resolution of a special electron microscope: the cryoTitan (of FEI Company). This enabled them, as the first in their field of research, to make three-dimensional images of very rapidly frozen samples. These showed how the clusters in the solution nucleate into larger, unstructured nano-particles with an average diameter of around thirty nanometers. An organic surface applied by the researchers ensures that these nano-particles can grow into larger particles, in which crystalline regions can later form by ordering of the ions. The TU/e researchers also demonstrated a second function of the organic layer: it controls with great precision the direction in which the mineral can grow into a fully fledged biomineral. They now hope to show that the mechanism they have identified also applies to the formation of other crystalline biominerals, and perhaps even to other, inorganic materials. This is important for research into bone growth and bone-replacement materials. In addition it could be used in nanotechnology, to allow the growth of nano-particles to be controlled in the same way as seems to be the case in nature: through subtle interactions between organic and inorganic materials. About biomineralization Biomineralization is the formation of inorganic materials in a biological environment, as it is found in bones, teeth and shells. In this process the formation of the mineral is controlled with great precision by specialized organic biomolecules such as sugars and proteins. Although the underlying mechanisms have already been studied for a long time, the process is still not fully understood. A widely used strategy is the use of so-called biomimetic studies, in which the process of biomineralization is simulated by a simplified system in a laboratory. This allows parts of the mineralization process to be studied individually. With this approach and by using the unique electron microscope referred to above, Sommerdijk's research group in the chemical engineering and chemistry department at TU/e have been able to image the earliest stages of such a biomimetically controlled mineralization reaction. Original publication: Emilie M. Pouget et al.; "The initial stages of template-controlled CaCO3 formation revealed by Cryo-TEM"; Science, 13 March 2009
Other news from the department science
These products might interest you

NANOPHOX CS by Sympatec
Particle size analysis in the nano range: Analyzing high concentrations with ease
Reliable results without time-consuming sample preparation

Eclipse by Wyatt Technology
FFF-MALS system for separation and characterization of macromolecules and nanoparticles
The latest and most innovative FFF system designed for highest usability, robustness and data quality

DynaPro Plate Reader III by Wyatt Technology
Screening of biopharmaceuticals and proteins with high-throughput dynamic light scattering (DLS)
Efficiently characterize your sample quality and stability from lead discovery to quality control

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.