Revolution in lithium mining?
New membrane discovery makes possible cleaner lithium extraction
Researchers have developed a new process for sustainable lithium extraction, which could help to address the growing global demand for the metals used in electric vehicle batteries and renewable energy storage.
Current ways of getting lithium are bad for the environment and more sustainable approaches are hard to perform on a large scale, but scientists have developed new membranes to pull lithium directly out of salty lake water using electricity, leaving other metal ions behind.
Publishing their findings in Nature Water (12 Mar), the international group of researchers from the UK, France, and China reveal that the process offers a promising alternative to traditional lithium extraction methods.
Co-author Professor Melanie Britton, from the University of Birmingham, commented: “There is a critical demand for more-sustainable processes addressing the global challenges of mineral availability and clean water supply, which lead to a circular economy.
“We believe our findings could lead to more efficient and sustainable lithium extraction, which is crucial for the batteries powering everyday devices such as smartphones, laptops, and electric vehicles.”
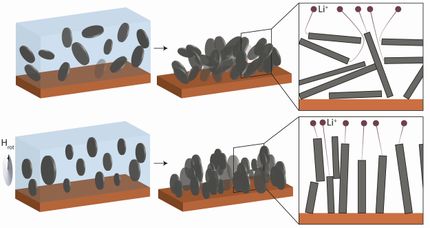
The new filtration membranes enable direct lithium extraction from salt-lake brines through a selective electrodialysis process – efficiently separating lithium ions from other ions present in the brine.
Dr. Qilei Song, from Imperial College London who lead the work, commented: "Our research could reduce the environmental impact of lithium mining and contribute to the development of more efficient energy storage systems for renewable energy sources. There may also be applications in other areas of resource recovery – for example, critical metal recovery from wastewater, plastic and battery recycling.”
These novel filters can tell the difference between ions with one electrical charge (monovalent) and those with two charges (divalent) - making them very good at separating different types of salt ion.
The membranes use very tiny channels, smaller than a nanometer (a billionth of a meter) lined with special chemical groups that interact with the ions as they pass through. PhD student Louie Lovell in Prof Britton’s team applied pulsed field gradient nuclear magnetic resonance (PFG-NMR) technique to characterize the water and ion diffusion in the subnanometer channels in the membranes.
They found that the water diffusion co-efficients strongly depend on the channel sizes and the chemical groups within the membranes. These membranes can produce very pure lithium carbonate (Li2CO3), which is good enough quality to be used in batteries.
Original publication
‘Solution-processable polymer membranes with hydrophilic subnanometre pores for sustainable lithium extraction’ - Dingchang Yang, Yijie Yang, Toby Wong, Sunshine Iguodala, Anqi Wang, Louie Lovell, Fabrizia Foglia, Peter Fouquet, Charlotte Breakwell, Zhiyu Fan, Yanlin Wang, Melanie M. Britton, Daryl R. Williams, Nilay Shah, Tongwen Xu, Neil B. McKeown, Maria-Magdalena Titirici, Kim E. Jelfs, and Qilei Song is published in Nature Water
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.