Lilly Receives Prestigious Award for Pioneering a Therapeutic Breakthrough with Xigris
Thursday, March 14, 2002
Eli Lilly and Company today received the Translational Medicine Award, which is co-sponsored by the University of California, the San Diego (UCSD) Institute of Molecular Medicine, The Salk Institute of Biological Studies and the journal Nature Medicine. The award was presented at the 2002 Days of Molecular Medicine Symposium and is in recognition of Lilly's new biotech product, Xigrisä (drotrecogin alfa (activated)). The award honors pioneering work in molecular medicine that leads to therapeutic advances for human diseases. Xigris also was recognized recently by Med Ad News as the Best New Medicine of 2001."Xigris is the first biologically targeted therapy for life-threatening severe sepsis, one of the most important unmet medical needs in all of medicine," said Kenneth R. Chien, M.D., Ph.D., director, UCSD Institute of Molecular Medicine. "Xigris has had a major clinical and scientific impact on an important human disease, which is why it was selected for this award."
"Our development of Xigris is more than just a significant scientific achievement,"
said August M. Watanabe, M.D., executive vice president, science and technology, for Lilly. "Since we introduced Xigris in the U.S. late last year, we've heard about many positive clinical outcomes from intensivists, infectious disease specialists and other physicians who have begun to use Xigris. Most importantly, we've heard from patients whose lives our therapy helped to save. We are humbled by the opportunity to make a difference in patients' lives."
About Xigris Before Xigris, there had been many failed attempts at bringing a severe sepsis treatment to market. After 20 years of research and development, Lilly was successful with the creation of one of the most complex biotech compounds ever produced. The Activated Protein C molecule is among the largest molecules used to make a biotech compound, measuring more than 400 amino acids in length as compared with, for example, the 51 amino-acid-length Humulinâ. The molecule has other specialized chemical attachments such as very complex carbohydrates that are not in other biotech treatments. Engineering a recombinant form of this molecule requires four complex modifications of the Protein C molecule to activate it to full biological potential.
Xigris was approved by the U.S. Food and Drug Administration in November 2001 for
the reduction of mortality in adult patients with severe sepsis (sepsis associated with acute organ dysfunction) who have a high risk of death (e.g., as determined by APACHE II¹). The efficacy of Xigris has not been established in adult patients with severe sepsis and lower risk of death. Safety and efficacy have not been established in pediatric patients with severe sepsis.
Bleeding events are common in patients with severe sepsis. Bleeding is the most
common adverse reaction associated with Xigris therapy. In the Phase III study, serious bleeding events were observed during the 28-day study period in 3.5 percent of Xigris-treated and 2.0 percent of placebo-treated patients. The difference in serious bleeding occurred primarily during infusion. Intracranial hemorrhage (ICH) may occur in patients with severe sepsis. In PROWESS, the incidence of intracranial hemorrhage was 0.2 percent for Xigris-treated and 0.1 percent for placebo-treated patients. ICH has been reported in Xigris-treated patients in non- placebo controlled trials with an incidence of approximately 1 percent during infusion. The risk of ICH may be increased in patients with risk factors for bleeding such as severe coagulopathy and severe thrombocytopenia.
Most read news
Topics
Organizations
Other news from the department research and development

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Chemists synthesize herbal alkaloid
LANXESS celebrates 75 years of production of ion exchange resins for the treatment of water
INEOS Olefins and Polymers to increase prices of its HDPE pipe grades in June
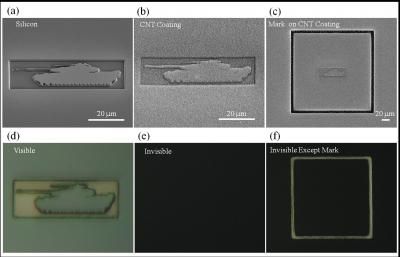
Carbon nanotube forest camouflages 3-D objects

Semi-liquid metal anode for next-generation batteries - New anode could help create a safer high energy lithium metal battery