Creating chiral carbons
South Korean scientists have developed a racemisation-resistant substrate that can be selectively alkylated to make new chiral carbon centres.
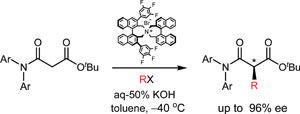
Mono-alkylation of a malonamic ester.
Although there are many ways to make chiral carbon centres by alkylating carbonyl compounds, until now scientists have been unable to asymmetrically mono-alkylate at the 2-position of 1,3-dicarbonyls because the compounds racemise easily under basic or acidic conditions.
Hyeung-geun Park, at Seoul National University, and colleagues converted one of the two ester groups on malonyl esters to an amide to reduce the acidity of the hydrogens at the 2-position. He showed that the resulting malonamic esters could be mono-alkylated under basic conditions at the 2-position with high enantioselectivity, indicating that the malonamic esters are racemisation-resistant.
'To the best of our knowledge, this is the first report to accomplish direct mono-alkylation of the 1,3-dicarbonyl system,' says Park. As well as reducing the acidity of the hydrogens, Park says he may have inhibited enolisation of the products by introducing strain between the N-substituent on the amide group and the new 2-substituent.
Chiral mono-alkylated malonyl derivatives are useful synthetic intermediates, explains Park, because they can be converted into diverse chiral building blocks. He has already selectively reduced the malonamic esters to give a range of products and plans to investigate more chemoselective transformations in the future.
Original publications: Mi-hyun Kim et al, Chem. Commun., 2009.
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.