MIT gel changes color on demand
Material could lead to fast, inexpensive sensors
MIT researchers have created a new structured gel that can rapidly change color in response to a variety of stimuli, including temperature, pressure, salt concentration and humidity. Among other applications, the structured gel could be used as a fast and inexpensive chemical sensor, says Edwin Thomas, MIT's Morris Cohen Professor of Materials Science and Engineering. One place where such an environmental sensor could be useful is a food processing plant, where the sensor could indicate whether food that must remain dry has been overly exposed to humidity.
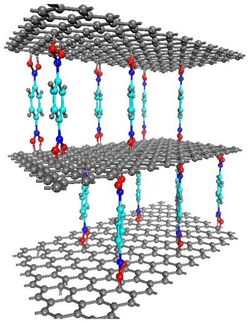
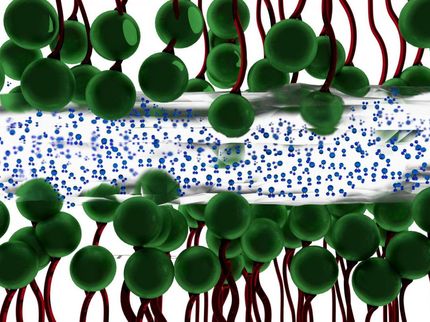
A critical component of the structured gel is a material that expands or contracts when exposed to certain stimuli. Those changes in the thickness of the gel cause it to change color, through the entire range of the visible spectrum of light. Objects that reflect different colors depending on which way you look at them already exist, but once those objects are manufactured, their properties can't change. The MIT team set out to create a material that would change color in response to external stimuli.
"We wanted to develop something that was 'tunable,'" said Thomas, who is head of MIT's Department of Materials Science and Engineering. To do that, they started with a self-assembling block copolymer thin film made of alternating layers of two materials, polystyrene and poly-2-vinyl-pyridine. The thickness of those layers and their refractive indices determine what color light will be reflected by the resulting gel. By keeping the thickness of the polystyrene layer constant and altering the thickness of the poly-2-vinyl-pyridine layer with external stimuli such as pH and salt concentration, the researchers were able to change the gel's color in fractions of a second.
"This is an ingenious and easy-to-implement method for making photonic materials whose optical properties can be readily tuned over a wide range," said Andrew Lovinger, director of the Polymers Program at the National Science Foundation, which funded this research.
The key to manipulating the thickness of the poly-2-vinyl-pyridine (2VP) layer is to give the nitrogens on each segment of the 2VP block a positive charge, yielding a polyelectrolyte chain that can swell to more than 1,000 percent its volume in water.
If the charges along the chain's backbone are electrically shielded from each other, for example by adding a high concentration of salt ions to the water that has permeated the gel, the 2VP chains collapse into disordered tangles, like balls of string. When the salt ions are washed away, the 2VP positive charges again repel each other and the chain extends, causing each 2VP layer to expand and the material to reflect a different color.
Because the diblock polymer film is a one-dimensional periodic stack, swelling is limited to one dimension, yielding a color shift of 575 percent in the reflected wavelength, a dramatic improvement over earlier color-changing gels that are made of charged colloids in a 3D lattice structure. Those gels expand in three dimensions, giving a much smaller range of color change.
The new gels are also sensitive to changes in pressure, humidity and temperature. "You can use mechanical or chemical forces to get really big responses, going through the entire range of light from ultraviolet (300 nanometers) to infrared" (1600 nm), Thomas said.
Original publication: Nature Materials 2007.
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.