Tiny Tubes and Rods Show Promise as Catalysts, Sunscreen
New ways to make, modify titanium oxide nanostructures for industrial, medical uses
Scientists at the U.S. Department of Energy's Brookhaven National Laboratory have developed new ways to make or modify nanorods and nanotubes of titanium oxide, a material used in a variety of industrial and medical applications. The methods and new titanium oxide materials may lead to improved catalysts for hydrogen production, more efficient solar cells, and more protective sunscreens. The research is published in two papers, one in Advanced Materials (August 22, 2007), and the other in the Journal of physical chemistry (September 8, 2007).
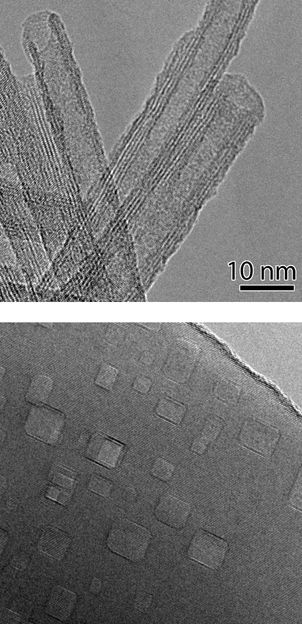
Transmission electron micrographs of nanocavity-filled titanium oxide nanorods (bottom) and iron-doped titanate nanotubes (top). Both are being investigated as photocatalysts for reactions to produce hydrogen gas. The improved light-absorption of the nanocavity-filled nanorods also makes them ideal new materials for sunscreen.
courtesy of DOE/Brookhaven National Laboratory
In the first study, the scientists enhanced the ability of titanium oxide to absorb light. "Titanium dioxide's ability to absorb light is one the main reasons it is so useful in industrial and medical applications," said Wei-Qiang Han, a scientist at Brookhaven's Center for Functional Nanomaterials (CFN) and lead author on both papers. It is used as a photocatalyst for converting sunlight to electricity in solar cells and also has applications in the production of hydrogen, in gas sensors, in batteries, and in using sunlight to degrade some environmental contaminants. It is also a common ingredient in sunscreen.
Many scientists have explored ways to improve the light-absorbing capability of titanium oxide, for example, by "doping" the material with added metals. Han and his coworkers took a new approach. They enhanced the material's light-absorption capability by simply introducing nanocavities, completely enclosed pockets measuring billionths of a meter within the 100-nanometer-diameter solid titanium oxide rods.
The resulting nanocavity-filled titanium oxide nanorods were 25 percent more efficient at absorbing certain wavelengths of ultraviolet A (UVA) and ultraviolet B (UVB) solar radiation than titanium oxide without nanocavities.
"Our research demonstrates that titanium oxide nanorods with nanocavities can dramatically improve the absorption of UVA and UVB solar radiation, and thus are ideal new materials for sunscreen," Han said. The cavity-filled nanorods could also improve the efficiency of photovoltaic solar cells and be used as catalysts for splitting water and also in the water-gas-shift reaction to produce pure hydrogen gas from carbon monoxide and water.
The method for making the cavity-filled rods is simple, says Han. "We simply heat titanate nanorods in air. This process evaporates water, transforming titanate to titanium oxide, leaving very densely spaced, regular, polyhedral nanoholes inside the titanium oxide."
In the second paper, Han and his collaborators describe a new synthesis method to make iron-doped titanate nanotubes, hollow tubes measuring approximately 10 nanometers in diameter and up to one micrometer long. These experiments were also aimed at improving the material's photoreactivity. The scientists demonstrated that the resulting nanotubes exhibited noticeable reactivity in the water-gas-shift reaction.
"Although the activity of the iron-doped nanotubes was not as good as that of titanium oxide loaded with metals such as platinum and palladium, the activity we observed is still remarkable considering that iron is a much less expensive metal and its concentration in our samples was less than one percent," Han said.
The scientists also observed interesting magnetic properties in the iron-doped nanotubes, and will follow up with future studies aimed at understanding this phenomenon.
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.
Last viewed contents

Process technology: SICK and Endress+Hauser sign strategic partnership - Companies to combine their process automation offerings at the turn of the year and establish a joint venture

labforward GmbH - Berlin, Germany

FlowLab Plus | Uniqsis