Tiny porphyrin tubes developed by Sandia may lead to new nanodevices
Research could result in clean, inexpensive hydrogen fuel
Advertisement
Sunlight splitting water molecules to produce hydrogen using devices too small to be seen in a standard microscope - That's a goal of a research team from the National Nuclear Security Administration's Sandia National Laboratories. The research has captured the interest of chemists around the world pursuing methods of producing hydrogen from water.
"The broad objective of the research is to design and fabricate new types of nanoscale devices," says John Shelnutt, Sandia research team leader. "This investigation is exciting because it promises to provide fundamental scientific breakthroughs in chemical synthesis, self-assembly, electron and energy transfer processes, and photocatalysis. Controlling these processes is necessary to build nanodevices for efficient water splitting, potentially enabling a solar hydrogen-based economy."
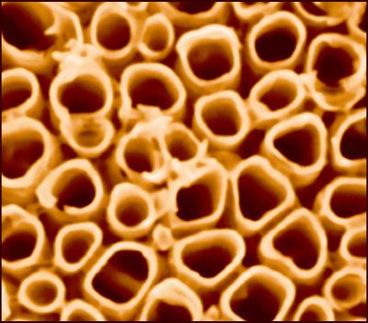
The prospect of using sunlight to split water at the nanoscale grew out of Shelnutt's research into the development of hollow porphyrin nanotubes. These light-active nanotubes can be engineered to have minute deposits of platinum and other metals and semiconductors on the outside or inside of the tube.
The key to making water-splitting nanodevices is the discovery by Zhongchun Wang of nanotubes composed entirely of porphyrins. Wang is a postdoctoral fellow at the University of Georgia working in Shelnutt's Sandia research group. The porphyrin nanotubes are micrometers in length and have diameters in the range of 50-70 nm with approximately 20 nm thick walls. They are prepared by ionic self-assembly of two oppositely charged porphyrins. These hollow structures are one member of a new class of nanostructures made of porphyrins that Shelnutt and his team are developing. The porphyrin building blocks (tectons) can be altered to control their structural and functional properties.
Shelnutt says these porphyrin nanotubes have "interesting electronic and optical properties such as an intense resonance light scattering ability and photocatalytic activity." When exposed to light, some porphyrin nanotubes can photocatalytically grow metal structures onto tube surfaces to create a functional nanodevice. For example, when the nanotubes are put into a solution with gold or platinum ions and exposed to sunlight, their photocatalytic activity causes the reduction of the ions to the metal. Using this method the researchers have deposited platinum outside the nanotube and grown a nanowire of gold inside the tube.
The nanotube with the gold inside and platinum outside is the heart of a nanodevice that may split water into oxygen and hydrogen. The research team has already demonstrated that the nanotubes with platinum particles on the surface can produce hydrogen when illuminated with light. To complete the nanodevice that splits water, a nanoparticle of an inorganic photocatalyst that produces oxygen must be attached to the gold contact ball that naturally forms at the end of the tube. The gold nanowire and ball serve as a conductor of electrons between the oxygen- and hydrogen- producing components of the nanodevice. The gold conductor also keeps the oxygen and hydrogen parts separate to prevent damage during operation.
"Laboratory-scale devices of this type have already been built by others," Shelnutt says. "What we are doing is reducing the size of the device to reap the benefits of the nanoscale architecture."
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.