A radical solution comes from mixing tools
For natural gas to be efficiently converted into useful industrial products requires the right catalytic process. Researchers from KAUST and the US combine state-of-the-art techniques for material characterization to demonstrate a unique reaction pathway that shows that molten catalysts based on sodium can provide all the chemical species required to optimize the process.
The active species in the catalytic reaction that exits on the molten surface of the sodium tungstate is sodium peroxide.
Reproduced with permission from reference 1 © 2017 Wiley-VCH Verlag GmbH & Co.
Free radicals, molecules with an unpaired valence electron, such as the hydroxyl radical, play a crucial role in the industrially important conversion of natural gas, primarily methane, to ethylene: a vital organic compound that forms the building blocks of many commodities and polymers. To enhance this process, known as oxidative coupling, it is vital to develop selective catalysts.
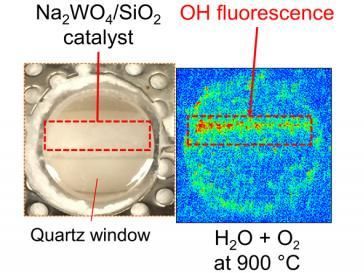
The KAUST team -- led by Kazuhiro Takanabe and his student Abdulaziz Khan -- used in situ tools to measure the state of a catalyst under reaction conditions. They discovered that the active species in the catalytic reaction that exits on the molten surface of the sodium tungstate, a chemical necessary for the reaction, is sodium peroxide. This catalyst is unique in that instead of activating methane directly, it firsts activates water and then generates gaseous hydroxyl radicals.
Oxidative coupling of methane converts methane and oxygen to ethylene in a single reactor. Previous research in Takanabe's lab had indicated that by using sodium tungstate at temperatures above 700 °C, the presence of water can both the increase the rate of methane conversion and enhance product selectivity. This could potentially occur via the formation of hydroxyl radicals and sodium peroxide, but there was no direct evidence for the presence of these species.
Now, Takanabe and his co-authors provide direct evidence for the formation of these free radicals on the molten surface of sodium tungstate. They combine a wide range of experimental techniques, including X-ray diffraction, scanning transmission electron microscopy, laser-induced fluorescence spectrometry and X-ray photoelectron spectroscopy, to observe an outer layer of molten sodium tungstate that is rich in sodium hydroxide. "We exclusively identified the catalyst's active phase in a unique state under reaction conditions," explains Takanabe.
This in turn confirms that a sodium-based catalyst can form hydroxyl radicals from a mixture of oxygen and water, a reaction that has never been seen. "This catalyst and the unique reaction pathway have great potential for use in various catalytic reactions for natural gas conversion, petroleum refinery and combustion reactions," says Takanabe.
More broadly, this success also demonstrates the importance of combining in situ spectroscopic and microscopic techniques to better understand high-temperature gas-phase chemistry.
Original publication
Kazuhiro Takanabe , Abdulaziz M. Khan , Yu Tang , Luan Nguyen , Ahmed Ziani , Benjamin W. Jacobs , Ayman M. Elbaz , S. Mani Sarathy , Franklin (Feng) Tao; "Integrated In Situ Characterization of a Molten Salt Catalyst Surface: Evidence of Sodium Peroxide and Hydroxyl Radical Formation"; Angew. Chem. Int. Ed.; 2017
Original publication
Kazuhiro Takanabe , Abdulaziz M. Khan , Yu Tang , Luan Nguyen , Ahmed Ziani , Benjamin W. Jacobs , Ayman M. Elbaz , S. Mani Sarathy , Franklin (Feng) Tao; "Integrated In Situ Characterization of a Molten Salt Catalyst Surface: Evidence of Sodium Peroxide and Hydroxyl Radical Formation"; Angew. Chem. Int. Ed.; 2017
Topics
Organizations
Other news from the department science
These products might interest you

Eclipse by Wyatt Technology
FFF-MALS system for separation and characterization of macromolecules and nanoparticles
The latest and most innovative FFF system designed for highest usability, robustness and data quality

Spinsolve Benchtop NMR by Magritek
Spinsolve Benchtop NMR
Spinsolve is a revolutionary multinuclear NMR spectrometer that provides the best performance

HYPERION II by Bruker
FT-IR and IR laser imaging (QCL) microscope for research and development
Analyze macroscopic samples with microscopic resolution (5 µm) in seconds

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.
Last viewed contents
Aromatic_hydrocarbon
G_protein
Nuclear_receptor
Ethenol
Liquification
GTPase
Sotalol
Clark_Heinrich
Category:Oxygen_heterocycles
Category:Zirconium
Chemokine





























































