Multifunctional catalyst for poison-resistant hydrogen fuel cells
Japanese collaboration develops catalyst that can oxidize both hydrogen and carbon monoxide to produce energy
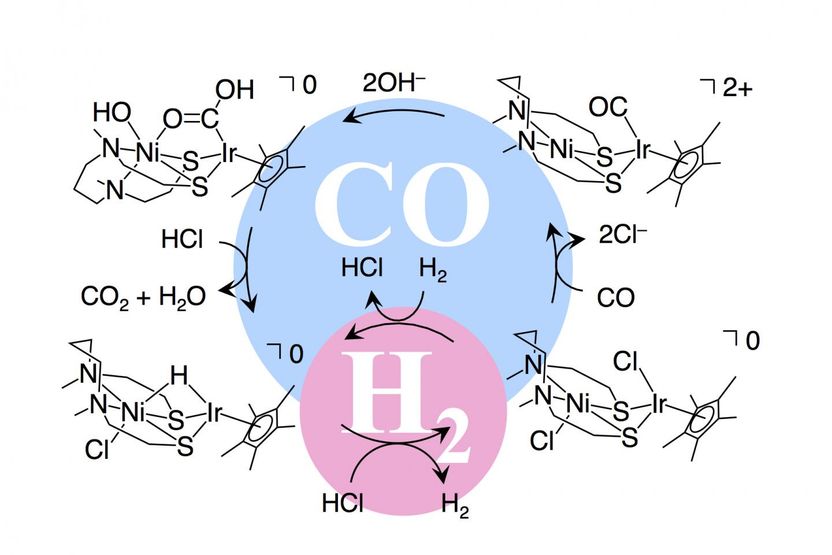
Reaction mechanism with a catalyst for fuel cells that use hydrogen and carbon monoxide as fuels.
Kyushu University
Demand for eco-friendly fuel sources is increasing as the goal of weaning off our reliance on fossil fuels becomes commonly recognized. Hydrogen represents a possible sustainable fuel source when it is produced from water and burned with oxygen because only water is released as a by-product. The oxidation of hydrogen to release energy and water using fuel cells containing catalysts is being researched intensively. However, catalysts used in hydrogen oxidation generally suffer from poisoning by carbon monoxide, which is present as a contaminant in commercial hydrogen gas. Thus, the ability to oxidize both hydrogen and carbon monoxide in the same reaction system is an attractive prospect to avoid catalyst poisoning and increase the efficiency of energy production from hydrogen.
A collaboration led by Kyushu University has recently developed a catalyst that can oxidize both hydrogen and carbon monoxide depending on the pH of the reaction system. The catalyst mimics the behavior of two enzymes: hydrogenase in acidic media (pH 4-7) and carbon monoxide dehydrogenase in basic media (pH 7-10). The catalyst is a water-soluble complex containing nickel and iridium metal atoms with a unique "butterfly" structure. The researchers investigated the ability of their catalyst to oxidize hydrogen, carbon monoxide, and a 1:1 mixture of these gases. Importantly, they were able to isolate various intermediates in the oxidation processes to confirm the mechanisms of hydrogen and carbon monoxide oxidation by the catalyst.
"We found that the catalyst reacted with hydrogen to form a hydride complex under acidic conditions," says first author Professor Seiji Ogo, Faculty of Engineering/ International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University. "In addition, the catalyst readily coordinated with carbon monoxide, which was oxidized to carbon dioxide, under basic conditions."
The team then investigated the resistance of their catalyst to poisoning by carbon monoxide in a prototype fuel cell using feed gases of hydrogen, carbon monoxide, and a 1:1 mixture of the two. The power density of the fuel cell containing the catalyst depended on the system pH and feed gas composition. Oxidation of hydrogen by the catalyst was facilitated at low pH (acidic conditions) and oxidation of carbon monoxide was faster at high pH (basic conditions); these trends correspond well with the behavior observed for the related enzymes.
"The ability of our catalyst to use both hydrogen and carbon monoxide as energy sources represents an important advance in hydrogen technology," explains Ogo.
It is anticipated that catalysts for hydrogen oxidation that can resist carbon monoxide poisoning will allow development of hydrogen fuels cells with improved performance, representing another step on the path towards the ultimate aim of a sustainable society.
Original publication
Other news from the department science
These products might interest you

Multi-Liter Hydrogen Gasgenerator by VICI
Laboratory hydrogen supply redefined
Up to 18 l/min hydrogen with 99.99997% purity and intuitive touchscreen control

CATLAB Catalysis and Thermal Analysis by Hiden Analytical
A system for catalyst characterisation, kinetic and thermodynamic measurements
Integrated Microreactor-Mass Spectrometer for Reaction Testing, TPD/R/O and Pulse Chemisorption.

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.

























































