The repulsion trick
A self-solving puzzle for organic molecules
Advertisement
Jülich researchers have succeeded in controlling the growth of organic molecules using a special trick. Molecules that repel each other play a key role in this process: due to their opposing forces, they always keep a certain distance from their neighbours. Therefore, they mix easily with a second, mutually attracting type of molecule that enters the spaces in-between and acts as a sort of "glue". Tailored surface structures can thus be put together like pieces in a puzzle - in a seemingly self-solving manner. Applications in the field of organic electronics in particular could stand to benefit from this method.
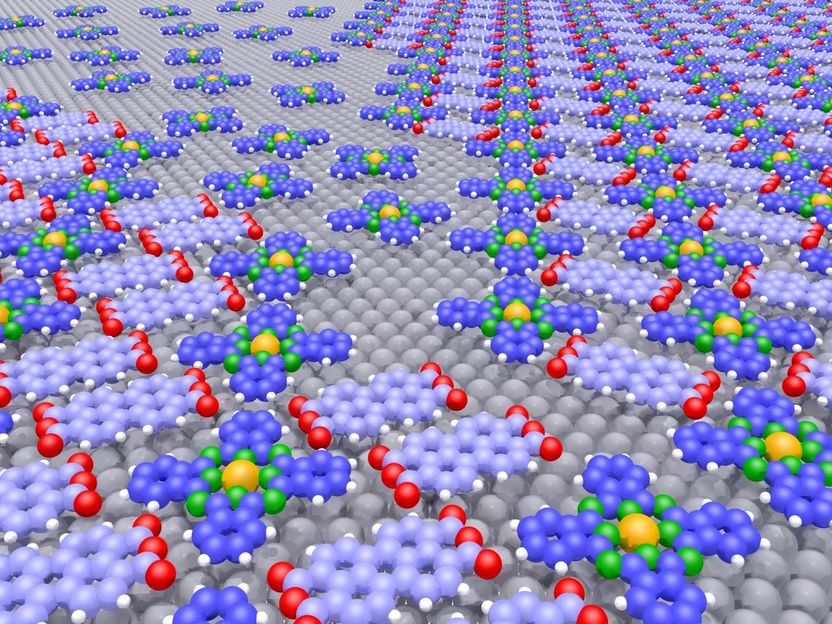
The figure shows two different crystalline structures (back right and front left) with a disordered area in between that only contains the repulsive molecular species. The sizes of the occupied areas can be altered (even at a later stage) by changing the mixing ratio, as electron-microscopic measurements have shown.
Forschungszentrum Jülich
Organic electronics is considered a pioneering technology of great promise. Organic light-emitting diodes, known as OLEDs, are today used all over the world. Further applications such as solar cells, sensors, and transistors are gradually finding their way into everyday use. However, as many fundamental correlations and processes have yet to be fully understood, these systems are still the subject of intensive ongoing research. In this context, the search for better mechanisms for the controlled and targeted production of active layer systems is one of the most important topics. Mixing molecules with opposing intermolecular interactions represents a possible new way of producing such structures in a targeted fashion.
Eutectic regions
In the system under study, the scientists at Forschungszentrum Jülich were able to observe three different monocrystalline mixed structures at different mixing ratios. Curiously, it is particularly interesting to study the system beyond the correct mixing ratio for these mixed crystalline phases. The scientists headed by Prof. Christian Kumpf from the Peter Grünberg Institute (PGI-3) found that in this case two phases coexist in equilibrium. In the phase diagram, this corresponds to eutectic regions, in which the equilibrium between the existing phases can be shifted in a large coverage regime by changing the mixing ratio, and thus the properties of the molecular layer can be tuned as desired.
In phase diagrams of conventional three-dimensional systems, usually no eutectic regions occur, but only eutectic points. This is, for example, the case for a number of metallic alloys, with soldering tin being a notable example. The large eutectic regions that occur in the heteromolecular layers investigated here are ultimately the result of the predefined size of the surface on which the molecules are adsorbed. The authors of the study were not only able to observe this behaviour experimentally, but also to explain it using fundamental thermodynamic considerations, and thus demonstrate that the existence of eutectic regions is a generic property of such two-dimensional mixed structures formed by molecules with opposing intermolecular interactions.