Expanding palette for color-changing glass
Rice University's latest nanophotonics research could expand the color palette for companies in the fast-growing market for glass windows that change color at the flick of an electric switch.
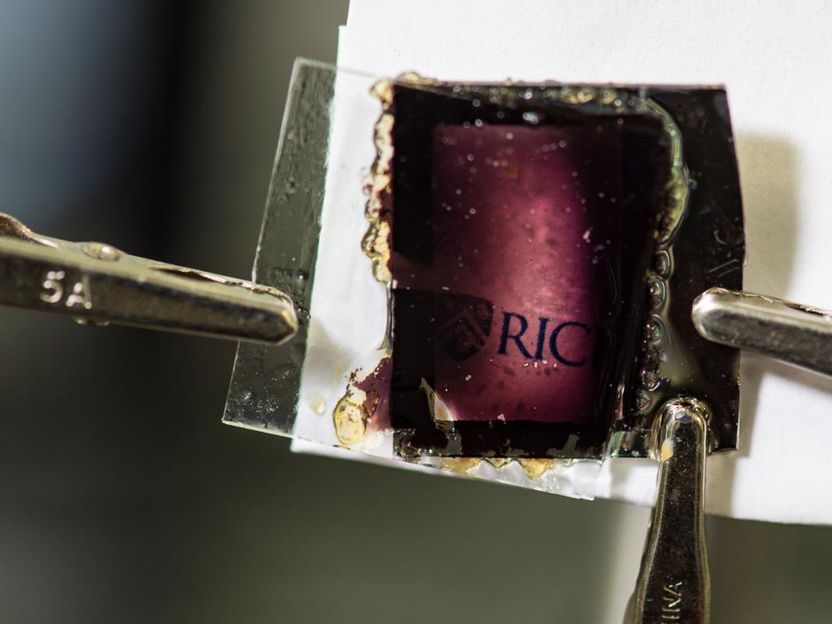
Rice University researchers demonstrated a new type of glass that turns from clear to black when a low voltage is applied. The glass uses a combination of molecules that block almost all visible light when they each gain a single electron.
Jeff Fitlow/Rice University
Researchers from the laboratory of Rice plasmonics pioneer Naomi Halas report using a readily available, inexpensive hydrocarbon molecule called perylene to create glass that can turn two different colors at low voltages.
"When we put charges on the molecules or remove charges from them, they go from clear to a vivid color," said Halas, director of the Laboratory for Nanophotonics (LANP), lead scientist on the new study and the director of Rice's Smalley-Curl Institute. "We sandwiched these molecules between glass, and we're able to make something that looks like a window, but the window changes to different types of color depending on how we apply a very low voltage."
Adam Lauchner, an applied physics graduate student at Rice and co-lead author of the study, said LANP's color-changing glass has polarity-dependent colors, which means that a positive voltage produces one color and a negative voltage produces a different color.
"That's pretty novel," Lauchner said. "Most color-changing glass has just one color, and the multicolor varieties we're aware of require significant voltage."
Glass that changes color with an applied voltage is known as "electrochromic," and there's a growing demand for the light- and heat-blocking properties of such glass. The projected annual market for electrochromic glass in 2020 has been estimated at more $2.5 billion.
Lauchner said the glass project took almost two years to complete, and he credited co-lead author Grant Stec, a Rice undergraduate researcher, with designing the perylene-containing nonwater-based conductive gel that's sandwiched between glass layers.
"Perylene is part of a family of molecules known as polycyclic aromatic hydrocarbons," Stec said. "They're a fairly common byproduct of the petrochemical industry, and for the most part they are low-value byproducts, which means they're inexpensive."
There are dozens of polycyclic aromatic hydrocarbons (PAHs), but each contains rings of carbon atoms that are decorated with hydrogen atoms. In many PAHs, carbon rings have six sides, just like the rings in graphene, the much-celebrated subject of the 2010 Nobel Prize in physics.
"This is a really cool application of what started as fundamental science in plasmonics," Lauchner said.
A plasmon is wave of energy, a rhythmic sloshing in the sea of electrons that constantly flow across the surface of conductive nanoparticles. Depending upon the frequency of a plasmon's sloshing, it can interact with and harvest the energy from passing light. In dozens of studies over the past two decades, Halas, Rice physicist Peter Nordlander and colleagues have explored both the basic physics of plasmons and potential applications as diverse as cancer treatment, solar-energy collection, electronic displays and optical computing.
The quintessential plasmonic nanoparticle is metallic, often made of gold or silver, and precisely shaped. For example, gold nanoshells, which Halas invented at Rice in the 1990s, consist of a nonconducting core that's covered by a thin shell of gold.
"Our group studies many kinds of metallic nanoparticles, but graphene is also conductive, and we've explored its plasmonic properties for several years," Halas said.
She noted that large sheets of atomically thin graphene have been found to support plasmons, but they emit infrared light that's invisible to the human eye.
"Studies have shown that if you make graphene smaller and smaller, as you go down to nanoribbons, nanodots and these little things called nanoislands, you can actually get graphene's plasmon closer and closer to the edge of the visible regime," Lauchner said.
In 2013, then-Rice physicist Alejandro Manjavacas, a postdoctoral researcher in Nordlander's lab, showed that the smallest versions of graphene -- PAHs with just a few carbon rings -- should produce visible plasmons. Moreover, Manjavacas calculated the exact colors that would be emitted by different types of PAHs.
"One of the most interesting things was that unlike plasmons in metals, the plasmons in these PAH molecules were very sensitive to charge, which suggested that a very small electrical charge would produce dramatic colors," Halas said.
Lauchner said the project really took off after Stec joined the research team in 2015 and created a perylene formulation that could be sandwiched between sheets of conductive glass.
In their experiments, the researchers found that applying just 4 volts was enough to turn the clear window greenish-yellow and applying negative 3.5 volts turned it blue. It took several minutes for the windows to fully change color, but Halas said the transition time could easily be improved with additional engineering.
Stec said the team's other window, which turns from clear to black, was produced later in the project.
"Dr. Halas learned that one of the major hurdles in the electrochromic device industry was making a window that could be clear in one state and completely black in another," Stec said. "We set out to do that and found a combination of PAHs that captured no visible light at zero volts and almost all visible light at low voltage."