Crystal clear imaging
Infrared brings to light nanoscale molecular arrangement
Detailing the molecular makeup of materials -- from solar cells to organic light-emitting diodes (LEDs) and transistors, and medically important proteins -- is not always a crystal-clear process.
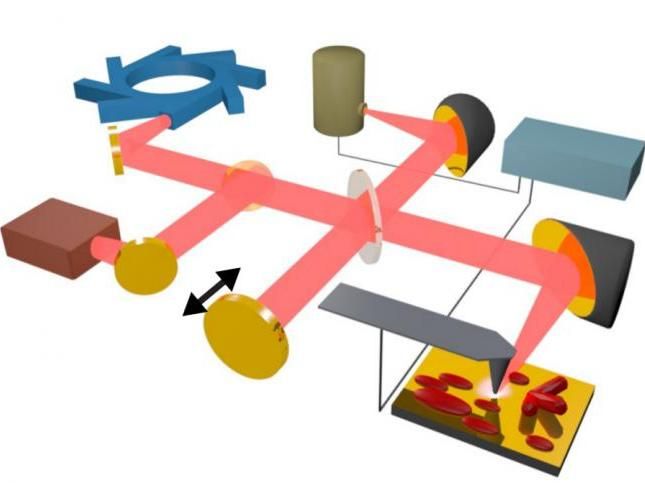
Infrared light (pink) produced by Berkeley Lab's Advanced Light Source synchrotron (upper left) and a conventional laser (middle left) is combined and focused on the tip of an atomic force microscope (gray, lower right), where it is used to measure nanoscale details in a crystal sample (dark red).
Berkeley Lab, CU-Boulder
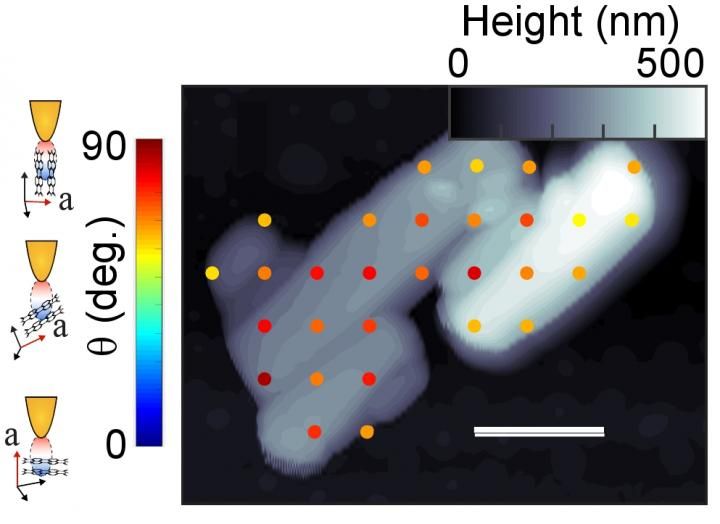
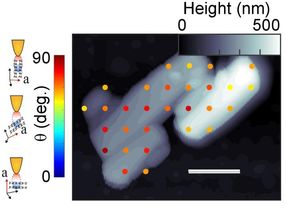
Researchers measured the molecular orientation of crystals (light gray and white) in samples of a semiconductor material known as PTCDA. The scale bar is 500 nanometers. The colored dots correspond to the orientation of the crystals in the color bar to the left. The figures at far left show the tip of the atomic force microscope in relation to different crystal orientations.
Berkeley Lab, CU-Boulder
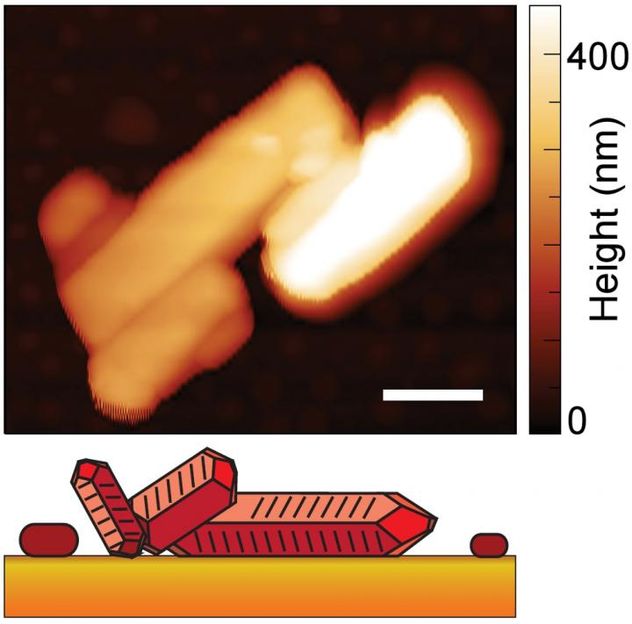
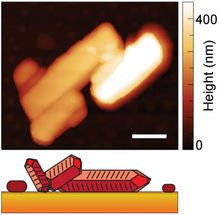
This image shows the crystal shape and height of a material known as PTCDA, with height represented by the shading (white is taller, darker orange is lowest). The white scale bar represents 500 nanometers. The illustration at bottom is a representation of the crystal shape.
Berkeley Lab, CU-Boulder
To understand how materials work at these microscopic scales, and to better design materials to improve their function, it is necessary to not only know all about their composition but also their molecular arrangement and microscopic imperfections.
Now, a team of researchers working at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) has demonstrated infrared imaging of an organic semiconductor known for its electronics capabilities, revealing key nanoscale details about the nature of its crystal shapes and orientations, and defects that also affect its performance.
To achieve this imaging breakthrough, researchers from Berkeley Lab's Advanced Light Source (ALS) and the University of Colorado-Boulder combined the power of infrared light from the ALS and infrared light from a laser with a tool known as an atomic force microscope. The ALS, a synchrotron, produces light in a range of wavelengths or "colors" -- from infrared to X-rays -- by accelerating electron beams near the speed of light around bends.
The researchers focused both sources of infrared light onto the tip of the atomic force microscope, which works a bit like a record-player needle -- it moves across the surface of a material and measures the subtlest of surface features as it lifts and dips.
The technique allows researchers to tune the infrared light in on specific chemical bonds and their arrangement in a sample, show detailed crystal features, and explore the nanoscale chemical environment in samples.
"Our technique is broadly applicable," said Hans Bechtel an ALS scientist. "You could use this for many types of material -- the only limitation is that it has to be relatively flat" so that the tip of the atomic force microscope can move across its peaks and valleys.
Markus Raschke, a CU-Boulder professor who developed the imaging technique with Eric Muller, a postdoctoral researcher in his group, said, "If you know the molecular composition and orientation in these organic materials then you can optimize their properties in a much more straightforward way.
"This work is informing materials design. The sensitivity of this technique is going from an average of millions of molecules to a few hundred, and the imaging resolution is going from the micron scale (millionths of an inch) to the nanoscale (billionths of an inch)," he said.
The infrared light of the synchrotron provided the essential wide band of the infrared spectrum, which makes it sensitive to many different chemicals' bonds at the same time and also provides the sample's molecular orientation. The conventional infrared laser, with its high power yet narrow range of infrared light, meanwhile, allowed researchers to zoom in on specific bonds to obtain very detailed imaging.
"Neither the ALS synchrotron nor the laser alone would have given us this level of microscopic insight," Raschke said, while the combination of the two provided a powerful probe "greater than the sum of its parts."
Raschke a decade ago first explored synchrotron-based infrared nano-spectroscopy using the BESSY synchrotron in Berlin. With his help and that of ALS scientists Michael Martin and Bechtel, the ALS in 2014 became the first synchrotron to offer nanoscale infrared imaging to visiting scientists.
The technique is particularly useful for the study and understanding of so-called "functional materials" that possess special photonic, electronic, or energy-conversion or energy-storage properties, he noted.
In principle, he added, the new advance in determining molecular orientation could be adapted to biological studies of proteins. "Molecular orientation is critical in determining biological function," Raschke said. The orientation of molecules determines how energy and charge flows across from cell membranes to molecular solar energy conversion materials.
Bechtel said the infrared technique permits imaging resolution down to about 10-20 nanometers, which can resolve features up to 50,000 times smaller than a grain of sand.
The imaging technique used in these experiments, known as "scattering-type scanning near-field optical microscopy," or s-SNOM, essentially uses the atomic force microscope tip as an ultrasensitive antenna, which transmits and receives focused infrared light in the region of the tip apex. Scattered light, captured from the tip as it moves over the sample, is recorded by a detector to produce high-resolution images.
"It's non-invasive, and it provides information about molecular vibrations," as the microscope's tip moves over the sample, Bechtel said. Researchers used the technique to study the crystalline features of an organic semiconductor material known as PTCDA (perylenetetracarboxylic dianhydride).
Researchers reported that they observed defects in the orientation of the material's crystal structure that provide a new understanding of the crystals' growth mechanism and could aid in the design molecular devices using this material.
The new imaging capability sets the stage for a new National Science Foundation Center, announced in late September, that links CU-Boulder with Berkeley Lab, UC Berkeley, Florida International University, UC Irvine, and Fort Lewis College in Durango, Colo. The center will combine a range of microscopic imaging methods, including those that use electrons, X-rays, and light, across a broad range of disciplines.
This center, dubbed STROBE for Science and Technology Center on Real-Time Functional Imaging, will be led by Margaret Murnane, a distinguished professor at CU-Boulder, with Raschke serving as a co-lead.
At Berkeley Lab, STROBE will be served by a range of ALS capabilities, including the infrared beamlines managed by Bechtel and Martin and a new beamline dubbed COSMIC (for "coherent scattering and microscopy"). It will also benefit from Berkeley Lab-developed data analysis tools.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!