Switch and stick
Some adhesives may soon have a metallic sheen and be particularly easy to unstick. Researchers at the Max Planck Institute for Intelligent Systems in Stuttgart are suggesting gallium as just such a reversible adhesive. By inducing slight changes in temperature, they can control whether a layer of gallium sticks or not. This is based on the fact that gallium transitions from a solid state to a liquid state at around 30 degrees Celsius. A reversible adhesive of this kind could have applications everywhere that temporary adhesion is required, such as industrial pick-and-place processes, transfer printing, temporary wafer bonding, or for moving sensitive biological samples such as tissues and organs. Switchable adhesion could also be suitable for use on the feet of climbing robots.
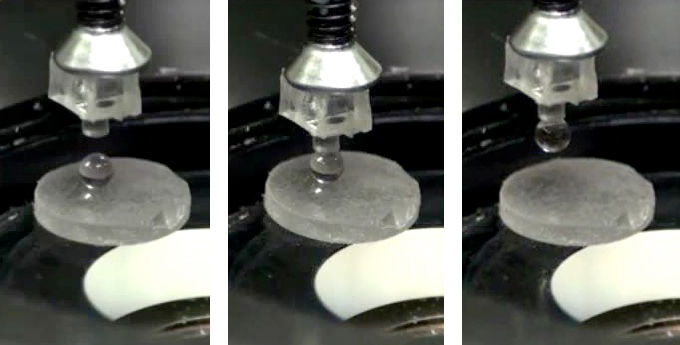
Stuttgart-based Max Planck researchers have used gallium as an adhesive to grip a glass sphere with a movable punch model. The metal is located at the bottom of the punch model. Once it touches the glass sphere, the researchers heat the gallium and then cool it off again, so that it connects to the glass. This way, they can retract the sphere from the surface.
© MPI f. Intelligente Systeme
The principle is actually quite simple: Above 30 degrees Celsius, gallium metal is liquid, and below 30 degrees it is solid. So if a drop of liquid gallium is introduced between two objects and then cooled to less than 30 degrees, the gallium layer solidifies and sticks the two objects together. When it is time to separate the objects, the temperature is raised to transition the gallium layer to its liquid state and they can be pulled apart with a small amount of unloading force. As an adhesive, gallium works in a similar fashion to hot glue, widely used in DIY applications. The difference is that far less heating and cooling are sufficient in the case of gallium, it lifts much more easily and cleanly from the surface, it is highly repeatable, and it is electrically conductive.
For their experiments, scientists working with Metin Sitti, Director at the Max Planck Institute for Intelligent Systems, wet the tip of a cylindrical elastomer rod with liquid gallium. They then brought the gallium droplet into contact with different materials such as glass, plastic and gold. After cooling the tip to 23 degrees, they found that the solidified gallium formed a strong bond between the elastomer and each of the materials.
Tests on particularly rough or damp surfaces
The researchers also took direct measurements of the effective binding power of gallium in both its liquid and solid phases. "The behaviour of these two values tells us something about the true reversibility and switchability of the adhesion process," explains Metin Sitti. The greater the difference of the binding power between the liquid and solid state, the easier it is to reverse and switch the adhesive effect.
The team deliberately tested gallium on particularly rough and damp surfaces as well. "These are surface conditions that showed up as major weaknesses of reversible micro/nanostructured adhesives proposed recently," says Sitti. How so? Adhesives that have yielded strong binding values on rough or wet surfaces to date have always had poor reversibility. Not so with the new gallium approach. The Stuttgart-based team have become convinced of its effectiveness in damp conditions, even testing it under water. Its binding power and reversibility when wet were reduced compared to dry conditions, but they still remained relatively strong for a wide range of applications.
Application wherever careful and reversible adhesion is required
Metin Sitti emphasises that gallium's performance in damp conditions makes it ideal for biological applications. The scientist and engineer foresees a time when gallium may be used to move individual cells, tissue samples or even organs, for example in laboratory or hospital settings.
Another possible field of application is industrial manufacturing, especially where fragile components such as ultra-thin graphene membranes or tiny electronic chips are involved. These components could be picked up by gallium-coated grippers and then set down at the precise location where they are required, e.g. a circuit board. In technical jargon, this kind of assembly technology is called "pick and place". It already exists today, but is generally based on the use of vacuum suction.
Metin Sitti believes the temperature-controlled gallium adhesive has two advantages. "Wetting an object with a metallic liquid such as gallium that forms a bond when cooled slightly is a far more gentle process for fragile materials than sucking them up using a vacuum," he expounds, adding that the new methods are also more energy-efficient. Once an object adheres to the gallium layer, no more energy is required to sustain the adhesive bond. Only when it is time to reverse the adhesion must the metal be quickly heated to 30 degrees. The vacuum technique, however, requires the constant use of suction in order to maintain the adhesive effect.
Temperature control for phase change of gallium
To achieve rapid heating and cooling as required in their tests, the team in Stuttgart connected a Peltier element to their experiment set-up. This element can either release or absorb heat when an electric current is applied. However, for practical applications in the future, the scientists anticipate that the adhesive bond could also be reversed using infrared radiation remotely or using electrical Joule heating by integrating conductive wiring to the surface.
Metin Sitti sees robotics as another possible application for this adhesive. For example, climbing robots such as those that may one day ascend wind turbines for maintenance purposes could benefit from reversible adhesives. By activating the adhesive, the robot foot would be fixed to the wall of the turbine, and for the next step, the adhesive layer between the foot and the wall would be briefly heated by means of an integrated heating element.
An adhesive that doesn't run out
A consideration of major importance for practical applications is that the material should be able to be used for as many cycles as possible without the need to replace it. Gallium conforms to this requirement, because the liquid metal lifts completely from the substrate with proper loading and unloading conditions. No residues are left on the surface, and the adhesive loses none of its own substance. This is by no means something to be taken for granted. "Good adhesives are generally hard to separate from the substrate," states Sitti, explaining that in gallium's case, the material forms a fine oxide layer in air. This shell of gallium oxide retains the gallium and ensures that no residues are left behind when the adhesion is reversed.
And that's not all. Gallium has even more to offer: "We can use it at different scales, from the nanometre range to microelectronics, and right up to larger applications," says Sitti with a smile. In theory, it could even be used to lift a fully-grown person as long as the contact surface was sufficiently large. However, it would be most cost-effective, energy efficient, and practical with smaller objects.
Metin Sitti believes that this method could be used in practical applications in the near future. And his team has started exploring some of these potential applications already. At the same time, they are working to optimize the technique. Until now, for example, the gallium was applied to an elastomer rod around two millimetres in diameter for all tests. "We want to test other elastomer geometries and designs with different length scales and see if we can enhance the binding strength as we do so," says Sitti. The scientists also plan to study alloys of gallium with other metals such as indium, but they will be watching closely to ensure that the melting point is close to normal ambient temperature.
Original publication
Zhou Ye, Guo Zhan Lum, Sukho Song, Steven Rich und Metin Sitti; "Phase Change of Gallium Enables Highly Reversible and Switchable Adhesion"; Advanced Materials; May 2016
































































