Challenging observations of 2-D melting and surface premelting at the single-particle level
The surface of a solid often melts into a thin layer of liquid even below its melting point. Such surface premelting is prevalent in all classes of solids; for instance, two pieces of ice can fuse below 0°C because the premelted surface water becomes embedded inside the bulk at the contact point and thus freeze. Premelting facilitates crystal growth and is critical in metallurgy, geology, and meteorology such as glacier movement, frost heave, snowflake growth and skating. However, the causative factors of various premelting scenarios, and the effect of dimensionality on premelting are poorly understood due to the lack of microscopic measurements.
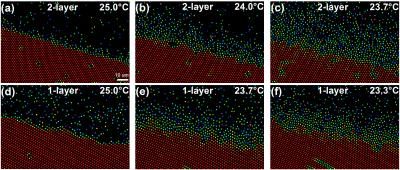
This image shows the surface premelting of bilayer (a-c) and monolayer (d-f) crystals. The color scale (shown in a) indicates the magnitude of the orientational-order parameter of each particle, |ψ6j|.
Department of Physics, HKUST
To this end, researchers from the Hong Kong University of Science and Technology (HKUST) and University of Amsterdam conducted a research where they were able to measure surface premelting with single-particle resolution for the first time by using novel colloidal crystals. They found that dimensionality is crucial to bulk melting and bulk solid-solid transitions, which strongly affect surface melting behaviors. To the surprise of the researchers, they found that a crystal with free surfaces (solid-vapor interface) melted homogenously from both surfaces and within the bulk, in contrast to the commonly assumed heterogeneous melting from surfaces. These observations would provide new challenges on premelting and melting theories.
Micrometer sized colloidal spheres in liquid suspensions have been used as powerful model systems for the studies of phase transitions because the thermal-motion trajectories of these "big atoms" can be directly visualized under an optical microscope. "Previous studies mainly used repulsive colloids, which cannot form stable solid-vapor interfaces," said Han. "Here, we made a novel type colloid with temperature-sensitive attractions which can better mimic atoms, since all atoms have attractions, or otherwise they cannot condense into stable solid in air. We assembled these attractive spheres into large well-tunable two-dimensional colloidal crystals with free surfaces for the first time.
"This paves the way to study surface physics using colloidal model systems. Our first project along this direction is about surface premelting, which was poorly understood before. Surprisingly, we found that it is also related to bulk melting and solid-solid transitions," Han added.
The team found that two-dimensional (2D) monolayer crystals premelted into a thin layer of liquid with a constant thickness, an exotic phenomenon known as incomplete blocked premelting. By contrast, the surface-liquid thickness of the two- or three-layer thin-film crystal increased to infinity as it approaches its melting point, i.e. a conventional complete premelting. Such blocked surface premelting has been occasionally observed, e.g. in ice and germanium, but lacks theoretical explanations.
"Here, we found that the premelting of the 2D crystal was triggered by an abrupt lattice dilation because the crystal can no longer provide enough attractions to surface particles after a drop in density." Li said. "Before the surface liquid grew thick, the bulk crystal collapsed and melted due to mechanical instability. This provides a new simple mechanism for blocked premelting. The two-layer crystals are mechanically stable because particles have more neighbors. Thus they exhibit a conventional surface melting."
As an abrupt dilation does not change the lattice symmetry, this is an isostructural solid-solid transition, which usually occurs in metallic and multiferroic materials. The colloidal system provides the first experimental observation of isostructural solid-solid transition at the single-particle level.
The mechanical instability induced a homogenous melting from within the crystal rather than heterogeneous melting from the surface. "We observed that the 2D melting is a first-order transition with a homogeneous proliferation of grain boundaries, which confirmed the grain-boundary-mediated 2D melting theory." said Han. "First-order 2D melting has been observed in some molecular monolayers, but the theoretically predicted grain-boundary formation has not been observed before."