Innovative catalyst fabrication method may yield breakthrough in fuel cell development
Advertisement
The successful future of fuel cells relies on improving the performance of the catalysts they use. gold nanoparticles have been cited as an ideal solution, but creating a uniform, useful catalyst has proven elusive. However, a team of researchers at Kyushu University's International Institute for Carbon-Neutral Energy Research (I2CNER) devised a method for using a new type of catalyst support.
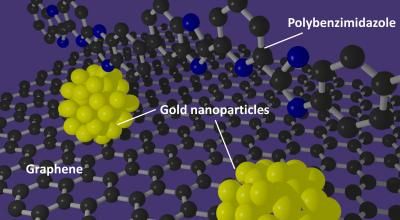
A polybenzimidazole polymer supports the formation of gold nanoparticles with well-defined sizes on graphene.
International Institute for Carbon-Neutral Energy Research (I²CNER), Kyushu University
In a potential breakthrough technology for fuel cells shows how wrapping a graphene support in a specially prepared polymer provides an ideal foundation for making uniform, highly active gold nanoparticle catalysts.
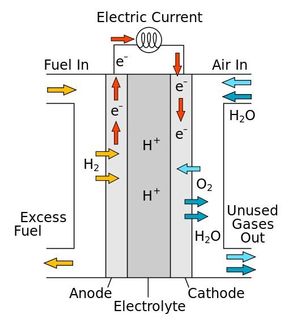
Fuel cells produce electricity directly from the separate oxidation of the fuel and the reduction of oxygen. The only by-product of the process is water, as fuel cells produce no greenhouse gases and are widely seen as essential for a clean-energy future.
However, the rate at which electricity can be produced in fuel cells is limited, especially by the oxygen reduction reaction (ORR), which must be catalyzed in practical applications. Although current platinum-based catalysts accelerate the reaction, their unhelpful propensity to also catalyze other reactions, and their sensitivity to poisoning by the reactants, limits their overall utility. Despite bulk gold being chemically inert, gold nanoparticles are surprisingly effective at catalyzing the oxygen reduction reaction without the drawbacks associated with their platinum counterparts.
Nevertheless, actually creating uniformly sized gold nanoparticle catalysts has proven problematic. Previous fabrication methods have produced catalysts with nanoparticle sizes that were too large or too widely distributed for practical use. Meanwhile, efforts to regulate the particle size tended to restrict the gold's activity or make less-stable catalysts.
"Creating small, well-controlled particles meant that we needed to focus on particle nucleation and particle growth," lead and corresponding author Tsuyohiko Fujigaya says. "By wrapping the support in the polybenzimidazole polymer we successfully developed with platinum, we created a much better support environment for the gold nanoparticles."
The team also tested the performance of these novel catalyst structures. Their catalysts had the lowest overpotential ever reported for this type of reaction. "The overpotential is a bit like the size of the spark you need to start a fire," coauthor Naotoshi Nakashima says. "Although we're obviously pleased with the catalysts' uniformity, the performance results show this really could be a leap forward for the ORR reaction and maybe fuel cells as well."