New Processes in Modern ReRAM Memory Cells Decoded
Resistive memory cells or ReRAMs for short are deemed to be the new super information-storage solution of the future. At present, two basic concepts are being pursued, which, up to now, were associated with different types of active ions. But this is not quite correct, as Jülich researchers working together with their Korean, Japanese and American colleagues were surprised to discover. In valence change memory (VCM) cells, not only are negatively charged oxygen ions active, but – akin to electrochemical metallization memory (ECM) cells – so too are positively charged metal ions. The effect enables switching characteristics to be modified as required and makes it possible to move back and forth from one concept to the other.
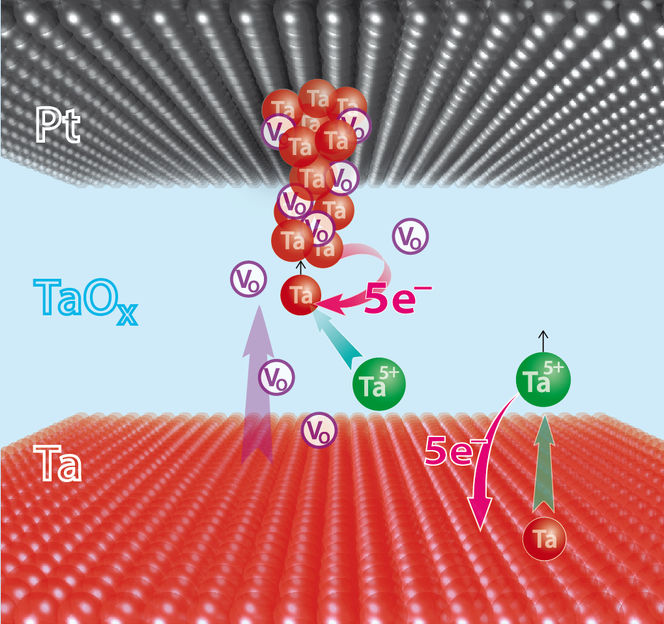
Formation of metallic Tantalum (Ta) filament within Ta/TaO(x)/Pt ReRAM memory cell. Positively charged Ta(5+)-ions and oxygen vacancies (V(O)) contribute to the process.
Copyright: Forschungszentrum Jülich / RWTH Aachen / Pössinger
ReRAM cells have a unique characteristic: their electrical resistance can be altered by applying an electric voltage. The cells behave like a magnetic material that can be magnetized and demagnetized again. In other words, they have an ON and an OFF state. This enables digital information to be stored, i.e. information that distinguishes between "1" and "0". The most important advantages of ReRAMs are that they can be switched rapidly, consume little energy, and maintain their state even after long periods of time with no external voltage.
The memristive behaviour of ReRAMs relay on mobile ions. These ions move in a similar manner to in a battery, flowing back and forth between two electrodes in a metal oxide layer no more than a few nanometres thick. For a long time, researchers believed that VCMs and ECMs functioned very differently. In ECMs, the ON and OFF states are achieved when metal ions move and form whisker-like filaments. This happens when an electric voltage is applied, causing such filaments to grow between the two electrodes of the cell. The cell is practically short-circuited and the resistance decreases abruptly. When the process is carefully controlled, information can be stored. The switching behaviour of VCMs, in contrast, were primarily associated with the displacement of oxygen ions. Contrary to metal ions, they are negatively charged. When a voltage is applied, the ions move out of an oxygen-containing metal compound. The material abruptly becomes more conductive. In this case as well, the process needs to be more carefully controlled.
Jülich researchers working together with their partners from the Chonbuk National University, Jeonju, the National Institute for Materials Science in Tsukuba and the Massachusetts Institute of Technology (MIT) in Boston discovered an unexpected second switching process in VCMs: metal ions also help to form filaments in VCMs. The process was made visible because the scientists suppressed the movement of the oxygen ions. To do so, they modified the surface by applying a thin carbon layer directly at the interface of the electrode material with the solid electrolyte. In one case, they used the "miracle material" graphene, which comprises only one single layer of carbon. "Graphene was used to suppress the transport of oxygen ions through the phase boundary and to slow down the oxygen reactions. Suddenly, we observed a switching characteristic similar to that of an ECM cell and therefore assume that free metal ions are also active in VCMs. This was additionally verified using scanning tunnelling microscopy (STM) and diffusion experiments. It appears that the metal ions provide additional support for the switching process," says Dr. Ilia Valov, electrochemist at Jülich’s Peter Grünberg Institute (PGI-7).
Incorporating such a carbon interlayer would make it possible to jump from one switching process to the other in VCMs. This would lead to new options for designing ReRAMs. "Depending on the application, our findings could be exploited and the effect purposely enhanced or intentionally suppressed," says Valov. The scientists’ findings give rise to several questions. "Existing models and studies will have to be reworked and adapted on the basis of these findings," says the Jülich scientist. Further tests will clarify how such novel components behave in practice.
Original publication
Most read news
Original publication
Anja Wedig, Michael Luebben, Deok-Yong Cho, Marco Moors, Katharina Skaja, Vikas Rana, Tsuyoshi Hasegawa, Kiran K. Adepalli, Bilge Yildiz, Rainer Waser, Ilia Valov; Nanoscale cation motion in TaOx, HfOx and TiOx memristive systems; Nature Nanotechnology (published 28 September 2015)
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Protein_efficiency_ratio
Efflux_(microbiology)