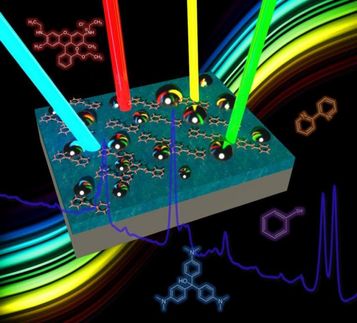
Wanted: The faces of the chemical crowd
Elements and their compounds will no longer be able to hide in mixtures, even if the latter are made up of many components. The end of chemical incognito is a result of the development at Warsaw's Polish Academy of Science's Institute of Physical Chemistry of a new, much more accurate method of identifying the "fingerprints" of chemical substances, imprinted in the light dispersed by the mixtures.
Elements and chemical compounds exist in their pure form only in laboratories. The world of nature is a world of multi-component additives and mixtures. Can individual components be precisely detected in the crowd of chemicals that make up a mixture? Can this be done purely by examining the light scattered by the mixture? To date, not only did current methods of spectral analysis not determine all of the components, but they even suggested the presence of compounds that were simply not there. However, now detection of the components of a mixture will be much more reliable - thanks to researchers at the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw. The research they have carried out, funded by the "Quantum semiconductor nanostructures" grant under the Innovative Economy Operational Programme for 2007-2013, has resulted in the development of a new algorithm for spectral analysis, with a much greater accuracy than the methods so far used.
Light carries information about the structure of matter - about the atoms and molecules with which it has interacted. Analysis of the radiation emitted or absorbed by the test substance is an excellent way of determining its chemical composition. All you need to know is how the desired compound interacts with light, and to detect a similar trace in the analyzed light. In theory, it's simple. In reality, there is a world filled with complex mixtures of various chemical compounds. Signals from all of them overlap and the detection of specific elements and compounds becomes extremely difficult.
"When you talk to someone face to face, you have no trouble understanding the message. But when you try to pick out the voice of a friend in a crowd of fans at a rock concert... The situation is similar for scientists dealing with spectroscopy: they are trying to pick out only the light 'shouts' of friends from the 'screams' of the crowd of chemical compounds. As if that were not enough, some friends can quite unexpectedly start speaking more quietly, or even go hoarse," says Dr. Sylwester Gawinkowski (IPC PAS).
If the only problem was the overlapping of "light screams", making out the signature of a specific chemical substance would not be especially difficult. Matters, however, become extremely complicated when it comes to the actual measurements. Inevitable measurement errors - including those arising from the physics itself of interaction of light with matter - mean that the registered radiation, even if it comes from only one substance, always has a slightly different structure to the theoretical pattern. In addition, it doesn't have to be one compound that is being sought, but several from a known pool. As if that were not enough, the contribution of each of the compounds in the structure of the analysed light may be different - In the case of one substance weaker, in the case of another stronger.
"When examining the structure of the light that is registered - that is, in the course of spectral analysis - the whole trick is to pick out only the most important elements characteristic of a given substance from the spectrum of the mixture. This approach is somewhat similar to the automatic face detection method used, for example, in security systems at airports. This does not involve a comparison of the appearance of every detail of the face, but a search for similarities in simple relationships, such as eye spacing, the position of the mouth or the end of the nose. Then it no longer matters if the wanted person is wearing a hat or not, or whether he has a suntan or has shaved off his moustache," explains Dr. Tomasz Roli?ski (IPC PAS).
Following this idea, physicists at IPC PAS have developed a method of analysis which is sensitive not to the intensity of the spectral lines of the light passing through the mixture, but to their relative positions and shapes. To test its efficacy, a series of tests was conducted. One of the researchers prepared mixtures of several amino acids randomly selected from a known pool of 20 compounds. The number of components of each mixture ranged from two to eight, but the volumes of the components were similar (which does not mean that all the substances reacted as strongly as each other with the light!). The mixture thus prepared was analysed by another researcher. The test mixtures first underwent spectral analysis using the current, popular method of least squares. In 7 cases out of 20 one component was not found, in one case even two. Moreover, in two mixtures the analysis detected the presence of two amino acids which were not even there.
"In the case of our method the results of analyses of the same spectra were qualitatively better," stresses Dr. Roli?ski and clarifies: "In 20 of the tested mixtures we correctly identified the composition in 18 cases. In the other two mixtures, one made up of five components, the other of eight, we did not detect a single component. Not once did we identify a compound that was not present in the mixture."
The new method of spectral analysis of mixtures can be applied, among others, in such sophisticated research techniques as Surface Enhanced Raman Spectroscopy (SERS). The specific nature of SERS spectroscopy results from the fact that the signals emitted by the chemical molecules can be enhanced hundreds of thousands or even millions of times. Such a level of enhancement permits us to consider designing detectors capable of detecting single chemical molecules.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!