Shaping the future of energy storage with conductive clay
Advertisement
In the race to find materials of ever increasing thinness, surface area and conductivity to make better performing battery electrodes, a lump of clay might have just taken the lead. Materials scientists from Drexel University's College of Engineering invented the clay, which is both highly conductive and can easily be molded into a variety of shapes and sizes. It represents a turn away from the rather complicated and costly processing - currently used to make materials for lithium-ion batteries and supercapacitors - and toward one that looks a bit like rolling out cookie dough with results that are even sweeter from an energy storage standpoint.
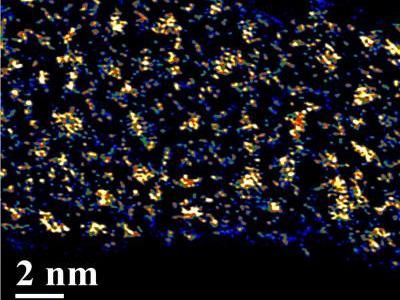
MXene clay, created by researchers at Drexel University, exhibits high volumetric capacitance through more than 10,000 charge/discharge cycles in early testing.
Drexel University
With the publication of their recipe for "conductive MXene clay" in Nature, the researchers suggest a significant shift in the way electrodes for storage devices are produced. The clay, which already exhibits conductivity on par with that of metals, can be turned into a film - usable in an electrode - simply by rolling or pressing it.
"Both the physical properties of the clay, consisting of two-dimensional titanium carbide particles, as well as its performance characteristics, seem to make it an exceptionally viable candidate for use in energy storage devices like batteries and supercapacitors," said Yury Gogotsi, PhD, Distinguished University and Trustee Chair professor in the College of Engineering, and director of the A.J. Drexel Nanomaterials Institute, who is a co-author of the paper. "The procedure to make the clay also uses much safer, readily available ingredients than the ones we used to produce MXene electrodes in the past."
The key to the utility of this material, according to Michel Barsoum, PhD, Distinguished professor in the College of Engineering and one of the inventors of MXenes, is in its form.
"As anybody who has played with mud can attest, clay is hydrophilic -water-loving," Barsoum said. "Clay is also layered and when hydrated, the water molecules slide between the layers and render it plastic that in turn can be readily shaped into complex shapes. The same happens here; when we add water to MXene, water penetrates between the layers and endows the resulting material with plasticity and moldability. Graphene - a material widely studied for use in electrodes- on the other hand, is conductive but does not like water - it is hydrophobic. What we discovered is a conductive two-dimensional layered material that also loves water. The fact that we can now roll our electrodes rapidly and efficiently, and not have to use binders and/or conductive additives renders this material quite attractive from a mass production point of view."
The discovery came about while Michael Ghidiu, a doctoral student advised by Barsoum and Gogotsi in the Department of Materials Science and Engineering at Drexel, was testing a new method for making MXenes - two-dimensional materials invented at Drexel that are among the leading candidates for use in next-generation batteries and supercapacitors.
Straying slightly from the original chemical etching process pioneered at Drexel, which uses highly toxic hydrofluoric acid, Ghidiu instead used a fluoride salt and hydrochloric acid to etch aluminum out of a titanium-based, layered ceramic material called a MAX phase - also discovered at Drexel by Barsoum. These two ingredients, which are household names in chemistry class and are also much safer to handle than hydrofluoric acid, reduced the MAX phase to a pile of black particles. To stop the reaction and remove any residual chemicals, Ghidiu washed the material in water. But rather than finding the familiar layered MXene particles, he discovered that the etched sediment absorbed the water to form a clay-like material.
"We expected to find a slightly different material coming from the new process - but nothing like this," Ghidiu said. "We were just hoping for a safer, less expensive way to make MXenes, when something even better landed on the table."
One of the first tests the team performed on the clay was to see if it could be pressed into a thin layer while retaining its conductive properties - after all, its initial goal was to make a conductive film.
"Being able to roll clay into a film is quite a contrast in production time, safety and cost when compared to the two most common practices for making electrode materials," Ghidiu said. "Both the etching and peeling process used to make MXenes and a flaking, filtration and deposition method - like paper making - employ strong acids and costly, less common materials. The clay-making process is much simpler, quicker and safer."
With the new discovery, all these steps are avoided, greatly simplifying the processing. Now the researchers can simply etch the MAX phase, wash the resulting material and roll the resultant clay into films of various thicknesses.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.