X-ray science taps bug biology to design better materials
Bug spray, citronella candles, mosquito netting – most people will do anything they can to stay away from insects during the warmer months. But those creepy crawlers we try so hard to avoid may offer substantial solutions to some of life’s problems.

Most people know the caddisfly as the artificial bug on fly fishing lures.
Argonne National Laboratory
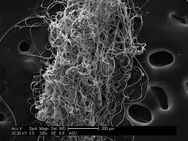
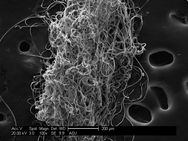
Caddiesflies spin an adhesive silk underwater to build nets to capture food and build protective shelter. Pictured is that silk magnified.
Bennett Addison

“(Caddisfly silk) is really not much stronger than super glue, but try to put super glue in your bathtub without it ever getting a chance to dry,” says Jeff Yarger, professor of chemistry, biochemistry and physics at Arizona State University.
Bennett Addison


Researchers using the cutting-edge X-ray technology at the U.S. Department of Energy’s Advanced Photon Source (APS) were able to take an inside look at several insects, gathering results that go beyond learning about insect physiology and biology. What they found could provide a blueprint for a material used for artificial ligaments, a chemical-free way to protect crops from insects and a new insight on how human muscles function.
Silk-spun tendons?
Most people know the caddisfly as the artificial bug on fly fishing lures. But few know that these real caddiesflies spin an adhesive silk underwater to build nets to capture food and build protective shelter. The chemical structure of the silk allows the substance to adhere to most substances underwater.
“It is really not much stronger than super glue, but try to put super glue in your bathtub without it ever getting a chance to dry,” says Jeff Yarger, professor of chemistry, biochemistry and physics at Arizona State University and author of a study in Biomacromolecules that examined caddisfly silk.
Designing a synthetic version of the silk could create an underwater adhesive used for liquid stitches. But even more valuable is its potential use as the first artificial human tendons and ligaments. The fly silk’s long fibers make it behave a lot like collagen material used in connective tissues, and its ability to adhere in wet conditions make it viable as an internal implant.
To understand what makes this material both waterproof and collagen-like, Yarger and his team had to examine the biopolymers, tiny molecular structures that serve as the building blocks for the silk, using the BioCARS sector 14 at the Argonne National Laboratory-based APS.
The crystalline structures in the silk are so small that Yarger says it is impossible to look at the molecular makeup of the silk with conventional X-rays. “But the synchrotron analysis at the APS allows us to do this,” Yarger said.
They found that at the molecular level, caddisfly silk differs greatly from other terrestrial spun silks such as those from spiders or silkworms. Caddisfly silk is phosphoratelated, meaning that after the amino acid chain that makes up the silk is created, phosphate molecules bond to the chain. Phosphates can act as bonding agents and are used to make some water resistant paints.
“The next step is to see how we might be able to mimic nature with this new motif we discovered,” Yarger says.
Putting grasshoppers on a diet
Grasshoppers eat up crops, but farmers may soon have a chemical-free way to protect their plants from these voracious pests by turning their natural growth cycle against them.
Scott Kirkton, associate professor of biology at Union College observed that just before molting, a growth process in which an insect sheds its skin in order to mature to its next life stage, a grasshoppers insides become essentially too large for its outer shell. This compresses the grasshopper’s tracheal system and makes it difficult for it to breathe. As a result, the team saw a reduction in the number of jumps per minute for the grasshoppers about to molt versus those that were not, suggesting that a compressed respiratory system causes a reduction in mobility.
From this, Kirkton hypothesizes that a lack of oxygen delivery to the grasshopper’s body is a trigger for molting. Storing grains or crops at low oxygen levels would limit the oxygen the insects get and trigger molting. The resulting stunted growth cycle would create petite pests with petite appetites, leaving more crops to make their way to supermarket shelves.
“A faster development time would produce smaller adults with reduced appetites and reduce the overall lifespan of the insect,” Kirkton said.
The key to discovering the connection between oxygen and the molting cycle came from Kirkton’s use of the phase-contrast beamline at sector 32 of the APS. That high-resolution imaging, rapid snapshot-like data collection and the ability to look deeply into material, created a unique ability to visualize and quantify the functioning respiratory system of an intact living insect in real time.
Kirkton published recently in the Journal of Comparative Physiology, his look at the respiratory system of the American grasshopper during periods right before molting. While Kirkton says that more research needs to be done, he thinks that this finding is applicable to a wide-range of insects, which means a universal and chemical-free pest control method may be on its way.
Muscled moths
Although few gym rats want to admit it, whispery moth wings and bulging human biceps aren’t that different. What we learn from them can teach us more about human muscle mechanics to potentially improve physical therapy treatments and further understand diseases attacking the muscular system.
But logistically, looking at the protein structures within a moth’s muscle cells is no easy task. The experiment setup involved gluing a moth by its thorax to a support structure, attaching a series of electrodes to its flight muscles to trigger its wings to beat at a rapid pace, and then using one of the world’s most powerful light sources to examine the molecular structure of its muscle movement in real time. The results shed light on more than the mechanics of moth flight – it may redefine our understanding of how our own muscles function.
To conduct this research, Tom Daniel, professor of biology at the University of Washington and author of a study in Science that examined the cross bridge cycling in the muscles of moths, had to seek out Thomas Irving. Irving is the director of the Biophysics Collaborative Access Team at sector 18 of the APS. Daniel says it is thanks to Irving’s wizardry – his expertise in biophysics and experience “hooking up insects to gizmos” – that helped Daniel pull together this experiment.
What they found was that when a moth flaps its wings, a bit of a tug of war is happening at a molecular level. Filaments of myosin tug on filaments of actin to contract a muscle strand, then detach to lengthen the strand. When connected and contracting, the filaments form a lattice-type structure, which is “rubbery,” and stores elastic energy. It’s like a microscopic trampoline, waiting for something to bounce on it. So when a muscle is contracting, it is acting more like a spring waiting to release its energy than a motor.
Using the APS, Daniel and his team observed that the top of the moth’s thorax, which is the muscle that makes the wings move, was cooler on top than on the bottom. The interesting part was that in the cooler regions, the filaments stayed connected for longer, maintaining the rubbery structure for a longer period of time. The elastic energy stored in these cooler regions is released at the end of the lengthening or shortening phases of the muscle. Think of it as a ball finally bouncing on that trampoline. This energy transfer process allows the moth to fly without expending a large amount of energy.
Daniel says that the presence of elastic energy was not a surprise.
“It was not a question of whether or not there was elastic energy involved in flight,” Daniel said. The energy cost of rapidly accelerating and decelerating wings during flight is enormous and no insect would be able to maintain that kind of energy output.
However, this study uncovers a new mechanism for this elastic energy storage, one based on temperature differences. At a molecular level, a moth’s muscle is not very different than a human’s, meaning that elastic energy may serve a much larger role in human muscle function than researchers previously thought.
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.