What is glass? A million-dollar question
Viscous when hot, and rigid when cold, glass poses one of physics' greatest mysteries: How does glass solidify from a liquid? What could we create if we better understood the properties of glassy materials? More advanced glasses could mean smartphones that wrap around the wrist or capsules that preserve drugs against temperature variation or flexible sheets of solar cells or membranes that pull salt out of seawater.
Three University of Akron researchers hoping to uncover the mysteries of glass formation have received a $1 million grant from the W.M. Keck Foundation. This is the first Keck Foundation grant issued to an Ohio research team in more than five years. The Foundation, which caps its research grants at $1 million, supports projects "that are distinctive and novel in their approach, question the prevailing paradigm, or have the potential to break open new territory in their field." The UA team joins the ranks of past grant recipients including Yale, Princeton, Caltech, Northwestern, Stanford and Columbia.
"What is the underlying physical origin of the glass transition? Whoever solves this will probably win a Nobel Prize," says Dr. David Simmons, assistant professor of polymer engineering at UA and principal investigator on the grant. With his colleagues, Alamgir Karim, associate dean for research, College of Polymer Science and Polymer Engineering and Goodyear Tire & Rubber Co. professor of polymer engineering, and Kevin Cavicchi, associate professor of polymer engineering, Simmons hopes to take a key step toward answering this question.
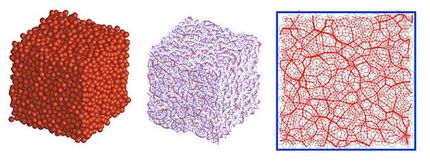
Comprised of molecules jammed and disordered, glasses lie in a mystery zone somewhere between liquids (which are comprised of mobile, disordered molecules) and crystals (which are made up of uniformly ordered and immobile molecules).
"The fundamental thing we don't understand is why glasses act as solids. Do they act like solids because they are really solid like most minerals and metals? Or, do they act like solids simply because they're very, very slow liquids? That's a very fundamental thing not to know about a material," Simmons says.
Windowpanes and wine goblets aside, glasses include many materials, such as plastics. Most plastic objects are really glasses made of polymers. There are biological glasses made from sugar used in medicine to encapsulate and preserve drugs. Consequently, discovering how glass transforms from a liquid to a solid has far-reaching impact on everything from your iPad to the vaccine you administer to a child.
How can we rationally and rapidly design glass-forming materials with exceptional properties?" asks Simmons. "We don't have systematic answers that allow us to predict how glasses will behave or how we can intervene in materials formation. This research can provide an algorithm that creates new and improved materials, mimicking the process of evolution over generations."
The scientists hope their algorithm will help develop glassy materials that are both impermeable and flexible, opening pathways to electronics-embedded thin films that can be folded and unfolded without damage, coatings that prevent bridges and roads from corrosion, and vaccines that can survive absence of refrigeration.
"We are using biomimicry — repurposing the process of evolution to evolve a better material," Simmons explains. "If we really understood how to design a glass with material properties we wanted, we could drive the next generation of advanced technologies, and push forward standard of living."
Simmons, an expert in simulation of polymers and glass formation, came to The University of Akron just over a year ago to work with the largest and most diverse concentration of polymer scientists. For this research grant, he is teamed with Karim, a world-renowned nanotechnology and properties-measurement scientist, and Cavicchi, an expert in materials synthesis. They combine their complementary expertise to learn how to evolve a new generation of materials to meet specific needs of industry and society.
"If you look around this campus, you have experts in all areas of polymers. I think this is the only place in the world that this proposal to the Keck Foundation could have been written," says Simmons.
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.