New material promises better solar cells
Researchers at the Vienna University of Technology show that a recently discovered class of materials can be used to create a new kind of solar cell.
Single atomic layers are combined to create novel materials with completely new properties. Layered oxide heterostructures are a new class of materials, which has attracted a great deal of attention among materials scientists in the last few years. A research team at the Vienna University of Technology, together with colleagues from the USA and Germany, has now shown that these heterostructures can be used to create a new kind of extremely efficient ultra-thin solar cells.
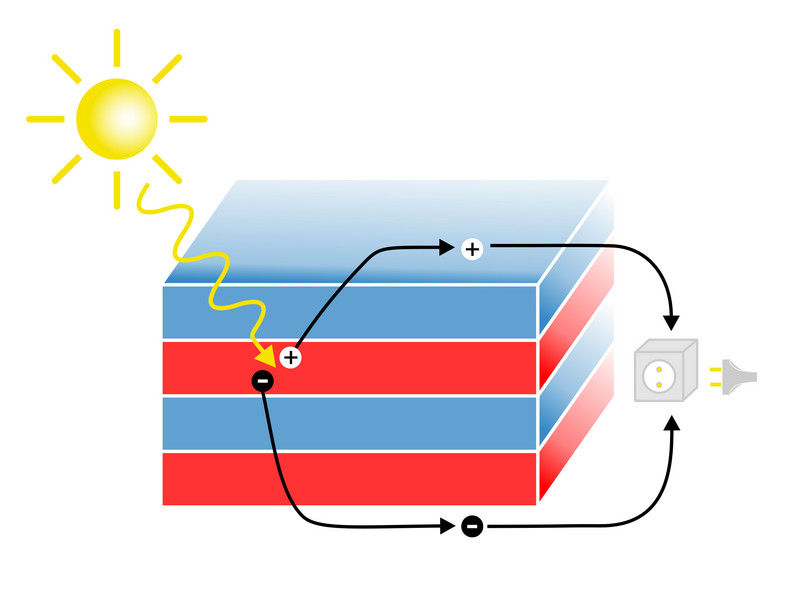
Sunlight is converted into electrical current in a layered structure.
Discovering new material properties in computer simulations
“Single atomic layers of different oxides are stacked, creating a material with electronic properties which are vastly different from the properties the individual oxides have on their own”, says Professor Karsten Held from the Institute for Solid State Physics, Vienna University of Technology. In order to design new materials with exactly the right physical properties, the structures were studied in large-scale computer simulations. As a result of this research, the scientists at TU Vienna discovered that the oxide heterostructures hold great potential for building solar cells.
Turning light into electricity
The basic idea behind solar cells is the photoelectric effect. Its simplest version was already explained by Albert Einstein in 1905: when a photon is absorbed, it can cause an electron to leave its place and electric current starts to flow. When an electron is removed, a positively charged region stays behind – a so called “hole”. Both the negatively charged electrons as well as the holes contribute to the electrical current.
“If these electrons and holes in the solar cell recombine instead of being transported away, nothing happens and the energy cannot be used”, says Elias Assmann, who carried out a major part of the computer simulations at TU Vienna. “The crucial advantage of the new material is that on a microscopic scale, there is an electric field inside the material, which separates electrons and holes.” This increases the efficiency of the solar cell.
Two isolators make a metal
The oxides used to create the material are actually isolators. However, if two appropriate types of isolators are stacked, an astonishing effect can be observed: the surfaces of the material become metallic and conduct electrical current. “For us, this is very important. This effect allows us to conveniently extract the charge carriers and create an electrical circuit”, says Karsten Held. Conventional solar cells made of silicon require metal wires on their surface to collect the charge carriers – but these wires block part of the light from entering the solar cell.
Not all photons are converted into electrical current with the same efficiency. For different colors of light, different materials work best. “The oxide heterostructures can be tuned by choosing exactly the right chemical elements”, says Professor Blaha (TU Vienna). In the computer simulations, oxides containing Lanthanum and Vanadium were studied, because that way the materials operate especially well with the natural light of the sun. “It is even possible to combine different kinds of materials, so that different colors of light can be absorbed in different layers of the solar cell at maximum efficiency”, says Elias Assmann.
Putting theory into practice
The team from TU Vienna was assisted by Satoshi Okamoto (Oak Ridge National Laboratory, Tennessee, USA) and Professor Giorgio Sangiovanni, a former employee of TU Vienna, who is now working at Würzburg University, Germany. In Würzburg, the new solar cells will now be build and tested. “The production of these solar cells made of oxide layers is more complicated than making standard silicon solar cells. But wherever extremely high efficiency or minimum thickness is required, the new structures should be able to replace silicon cells”, Karsten Held believes.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.

























































