A new catalyst for ethanol made from biomass
Researchers potentially find a renewable path to fuel additives, rubber and solvents
Researchers in the Pacific Northwest have developed a new catalyst material that could replace chemicals currently derived from petroleum and be the basis for more environmentally friendly products including octane-boosting gas and fuel additives, bio-based rubber for tires and a safer solvent for the chemicals industry.
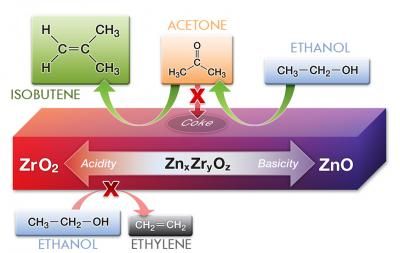
The right balance of zinc and zirconium oxides in this catalyst (purple block) converts ethanol to isobutene with low amounts of unwanted byproducts such as acetone and ethylene.
PNNL
To make sustainable biofuels, producers want to ferment ethanol from nonfood plant matter such as cornstalks and weeds. Currently, so-called bio-ethanol's main values are as a non-polluting replacement for octane-boosting fuel additives to prevent engine knocking and as a renewable replacement for a certain percentage of gasoline. To turn bio-ethanol into other useful products, researchers at the Department of Energy's Pacific Northwest National Laboratory and at Washington State University have developed a new catalyst material that will convert it into a chemical called isobutene. And it can do so in one production step, which can reduce costs.
Reported by researchers in the Institute for Integrated Catalysis at PNNL and in the Gene and Linda Voiland School of Chemical Engineering and Bioengineering at WSU, the findings appeared in the Journal of the American Chemical Society.
"Isobutene is a versatile chemical that could expand the applications for sustainably produced bio-ethanol," said chemical engineer Yong Wang, who has a joint appointment at PNNL in Richland, Wash. and at WSU in Pullman, Wash., and leads research efforts at both institutions.
In addition, this catalyst requires the presence of water, allowing producers to use dilute and cheaper bio-ethanol rather than having to purify it first, potentially keeping costs lower and production times faster.
No Z-Z-Z for the Weary
An important key to unlocking renewables to replace fossil fuel products is the catalyst. A catalyst is a substance that promotes chemical reactions of interest. The catalytic converter in a car, for example, speeds up chemical reactions that break down polluting gases, cleaning up a vehicle's exhaust.
The PNNL and WSU researchers were trying to make hydrogen fuel from ethanol. To improve on a conventional catalyst, they had taken zinc oxide and zirconium oxide and combined both into a new material called a mixed oxide -- the zinc and the zirconium atoms woven through a crystal of oxygen atoms. Testing the mixed oxide out, PNNL postdoctoral researcher Junming Sun saw not only hydrogen, but -- unexpectedly -- quite a bit of isobutene (EYE-SO-BEW-TEEN).
Hydrogen is great, but isobutene is better. Chemists can make tire rubber from it or a safer solvent that can replace toxic ones for cleaning or industrial uses. Isobutene can also be readily turned into jet fuel and gasoline additives that up the octane -- that value listed on gas pumps that prevents an engine from knocking -- such as ETBE.
Sun Shines
No one had ever seen a catalyst create isobutene from ethanol in a one-step chemical reaction before, so the researchers realized such a catalyst could be important in reducing the cost of biofuels and renewable chemicals.
Investigating the catalyst in greater depth, the researchers examined what happened when they used different amounts of zinc and zirconium. They showed that a catalyst made from just zinc oxide converted the ethanol mostly to acetone, an ingredient in nail polish remover. If the catalyst only contained zirconium oxide, it converted ethanol mostly to ethylene, a chemical made by plants that ripens fruit.
But the isobutene? That only arose in useful amounts when the catalyst contained both zinc and zirconium. And "useful amounts" means "a lot." With a 1:10 ratio of zinc to zirconium, the mixed oxide catalyst could turn more than 83 percent of the ethanol into isobutene.
"We consistently got 83 percent yield with improved catalyst life," said Wang. "We were happy to see that very high yield."
Reactionary Insight
The researchers analyzed the chemistry to figure out what was happening. In the single metal oxides experiments, the zinc oxide created acetone while the zirconium oxide created ethylene. The easiest way to get to isobutene from there, theoretically speaking, is to convert acetone into isobutene, which zirconium oxide is normally capable of. And the road from ethanol to isobutene could only be as productive as Sun found if zirconium oxide didn't get side-tracked turning ethanol into ethylene along the way.
Something about the mixed oxide, then, prevented zirconium oxide from turning ethanol into the undesired ethylene. The team reasoned the isobutene probably arose from zinc oxide turning ethanol into acetone, then zirconium oxide -- influenced by the nearby zinc oxide -- turning acetone into isobutene. At the same time, the zinc oxide's influence prevented the ethanol-to-ethylene conversion by zirconium oxide. Although that's two reaction steps for the catalyst, it's only one for the chemists, since they only had to put the catalyst in with ethanol and water once.
To get an idea of how close the reactions had to happen to each other for isobutene to show up, the team combined powdered zinc oxide and powdered zirconium oxide. This differed from the mixed oxide in that the zinc and zirconium atoms were not incorporated into the same catalyst particles. These mixed powders turned ethanol primarily into acetone and ethylene, with some amounts of other molecules and less than 3 percent isobutene, indicating the magic of the catalyst came from the microstructure of the mixed oxide material.
Balancing Act
So, the researchers explored the microstructure using instruments and expertise at EMSL, DOE's Environmental Molecular Sciences Laboratory on the PNNL campus. Using high-powered tools called transmission electron microscopes, the team saw that the mixed oxide catalyst was made up of nanometer-sized crystalline particles.
A closer look at the best-performing catalysts revealed zinc oxide distributed evenly over regions of zirconium oxide. The worst performing catalyst -- with a 1:1 zinc to zirconium ratio -- revealed regions of zinc oxide and regions of zirconium oxide. This suggested to the team that the two metals had to be close to each other to quickly flip the acetone into isobutene.
Experimental results from other analytical methods indicated that the team could optimize the type of chemical reactions that lead to isobutene and also prevent the catalyst from deactivating at the same time. The elegant balance of acidic and basic sites on the mixed oxides significantly reduced carbon from building up and gunking up the catalysts, which cuts their lifespan.
Future work will look into optimizations to further improve the yield and catalyst life. Wang and colleagues would also like to see if they can combine this isobutene catalyst with other catalysts to produce different chemicals in one-pot reactions.