The molecular pressure cooker
New method for producing nanostructures from molecules
Basic research in a research museum: a team of nanoscientists in the laboratories of the Deutsches Museum has developed a new method for producing stable molecular nanostructures on inert surfaces. The results of this work have now been published in the journal Angewandte Chemie International Edition.

Lukas Grossmann at the experimental setup in the nano lab of the Deutsches Museum.
Deutsches Museum

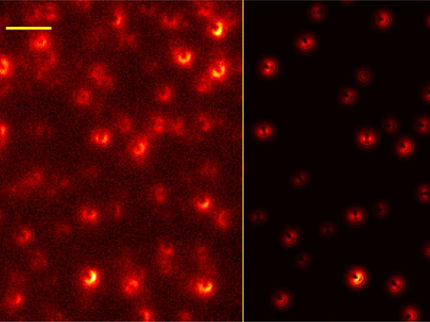
Nanostructures on graphite surface.
Deutsches Museum

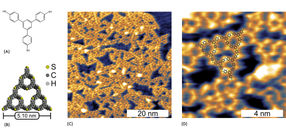
It looks a bit like a hobby cellar for tinkerers: shelves of tools on the wall, tables with strange apparatus, screws, pliers, electronic and mechanical accessories, a computer workstation, a tower with electronic components from different eras and, right in the middle, the actual experimental setup: a construct consisting of a vacuum chamber with various lines and control windows and a scanning tunneling microscope as the centerpiece. This is where Markus Lackinger, Head of the Laboratory for Nanosciences at the Deutsches Museum, and his postdoc Lukas Grossmann work.
Their field of research is called "OSS", On-Surface Synthesis: Specially designed molecules are deposited on a surface and then reacted by heating in an ultra-high vacuum in order to combine them to form nanostructures (synthesis). "In the conventional way, the molecules for such processes are applied to metal surfaces, which favor the reaction, but unfortunately also have tangible disadvantages," says Markus Lackinger.
For example, interactions with the underlying metal influence and change the properties of the resulting nanostructures, which are particularly relevant for applications. In addition, the metals - gold, silver or copper are most commonly used - ensure that the nanostructures are less stable because they not only facilitate the desired linking reaction, but also accelerate the decomposition of the networks and molecules. Metal surfaces are also very susceptible to contamination and oxidation. This is critical if the nanostructures are to be used in future outside the ultra-high vacuum in which they were produced.
About a year ago, Markus Lackinger and his colleague Lukas Grossmann therefore began experimenting with graphite as a substrate for molecular synthesis. "Graphite has no chemical effect on our reaction," explains Grossmann, "which means that the molecular nanostructures are created solely through the influence of temperature. This means that the graphite surface does not contribute to decomposition, which makes the nanostructures on graphite much more robust than on a metallic surface." And as the nanostructures only interact weakly with the graphite substrate, it would be easier to investigate their intrinsic properties in future and use them later.
But it wasn't quite that simple - replacing gold with graphite - after all: "When heating up, an important advantage of metals was missing - namely that they strongly bind the molecules on the surface," says Lackinger. "If the molecules were lying on the graphite, they would dissolve into thin air as the temperature rose." So to speak - because "air" is actually the wrong term in this case, as the synthesis normally takes place in a vacuum.
And this is precisely where the solution to the problem lay: "Our trick is that we don't heat in a vacuum, but in a noble gas atmosphere," says Markus Lackinger. "The argon atoms keep our molecules on the graphite surface long enough for them to react with each other at higher temperatures without flying away." Another trick was to increase the temperature around one hundred times slower than usual. This is the only way to give the molecules enough time to bond and stabilize at the reaction temperature.
The method even works on the surfaces of the miracle material graphene. This two-dimensional material, which is only one carbon atom thick, is even less reactive and particularly attractive for science due to its exotic properties: "Covalent molecular nanostructures on graphene could be the starting point for the production and research of new types of electronic components made from molecular nanostructures," says Markus Lackinger.
With the publication about the "molecular pressure cooker", as Markus Lackinger calls heating in noble gas, in the journal Angewandte Chemie International Edition, the results could also become the basis for further research work by other working groups. In the laboratories of the Research Museum on Munich's Museum Island, however, the scientists will first test their method with other molecules.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.