Less is more: Why an economical Iridium catalyst works so well
Different chemical environments explored
Iridium-based catalysts are needed to produce hydrogen using water electrolysis. Now, a team at HZB has shown that the newly developed P2X catalyst, which requires only a quarter of the iridium, is as efficient and stable over time as the best commercial catalyst. Measurements at BESSY II have now revealed how the special chemical environment in the P2X catalyst during electrolysis promotes the oxygen evolution reaction during water splitting.
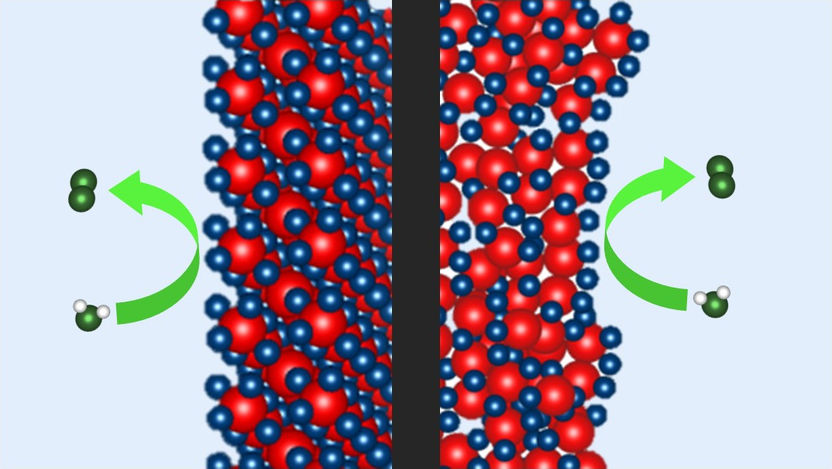
Two different Iridium-based nanocatalysts for water electrolysis were examined by a HZB-led team: A commercial benchmark catalyst (left) and the newly developed P2X catalyst (right), which is more amorphous and needs four times less Iridium. The spectroelectrochemical data show how the specific chemical environments differ in both materials and how these influence the oxygen evolution reaction.
© M. van der Merwe / HZB
In the future, hydrogen will be needed in a climate-neutral energy system to store energy, as a fuel, and a raw material for the chemical industry. Ideally, it should be produced in a climate-neutral way, using electricity generated from harnessing the sun’s or wind energy, via the electrolysis of water. In that respect, Proton Exchange Membrane Water Electrolysis (PEM-WE) is currently considered a key technology. Both electrodes are coated with special electrocatalysts to accelerate the desired reaction. Iridium-based catalysts are best suited for the anode, where the sluggish oxygen evolution reaction occurs. However, iridium is one of the rarest elements on earth, and one of the major challenges is to significantly reduce the demand for this precious metal. A rough analysis showed that to meet the world’s hydrogen demand for transport using PEM-WE technology, iridium-based anode materials should contain no more than 0.05 mgIr/cm2. The current, best commercially available catalyst made from iridium oxide contains about 40 times as much as this target value.
P2X-catalyst needs less Iridium
But new options are already in the pipeline: Within the Kopernikus P2X project, a new efficient iridium-based nanocatalyst was developed by the Heraeus Group, consisting of a thin layer of iridium oxide deposited on a nanostructured titanium dioxide support. The so-called 'P2X catalyst' requires only an extremely small amount of iridium, reducing precious metal loading substantially (four times lower than in the current best commercial material).
A team at HZB led by Dr. Raul Garcia-Diez and Prof. Dr.-Ing. Marcus Bär, together with colleagues from the ALBA synchrotron in Barcelona, have studied the P2X catalyst, which shows remarkable stability even in long-term operation, and compared its catalytic and spectroscopic signature with the benchmark commercial crystalline catalyst.
Operando measurements at BESSY II
The HZB team has thoroughly investigated the commercial benchmark catalyst as well as the P2X catalyst at BESSY II during water electrolysis (operando measurements). “We wanted to observe how the two different catalyst materials change structurally and electronically during the electrochemical oxygen evolution reaction using operando Ir L3-edge X-ray absorption spectroscopy (XAS),” says Marianne van der Merwe, a researcher in Bär's team. They also developed a new experimental protocol to ensure that the results are measured in both samples under exactly the same oxygen production rate. This made it possible to compare the two catalysts under equivalent conditions.
Different chemical environments explored
“From the measurement data, we were able to conclude that the mechanisms for OER in the two classes of iridium oxide catalysts are different, and this is driven by the different chemical environments of the two materials,” says van der Merwe. The measurement data also show why the P2X catalyst performs even better compared to its more crystalline benchmark: in the P2X sample, the bond lengths between iridium and oxygen decrease significantly more than in the reference catalyst at OER relevant potentials. This reduction in Ir-O bond lengths can be associated to the participation of defective environments that are proposed to be key players in highly active pathways of the oxygen evolution reaction.
“In addition, the electronic state observations also correlate with local geometric information”, van der Merwe points out. “Our work provides valuable key information about the different mechanisms of iridium oxide-based electrocatalysts during the oxygen evolution reaction and deepens our understanding of catalyst performance and stability, while our newly proposed in situ spectroscopic electrochemical protocol approach is generally applicable to all anode materials studied under relevant OER conditions”.
Original publication
Marianne van der Merwe, Romualdus Enggar Wibowo, Catalina E. Jimenez, Carlos Escudero, Giovanni Agostini, Marcus Bär, Raul Garcia-Diez; "Electronic and Structural Property Comparison of Iridium-Based OER Nanocatalysts Enabled by Operando Ir L3-Edge X-ray Absorption Spectroscopy"; ACS Catalysis, Volume 14, 2024-10-30
Maximilian Bernt, Alexandra Hartig‐Weiß, Mohammad Fathi Tovini, Hany A. El‐Sayed, Carina Schramm, Jonas Schröter, Christian Gebauer, Hubert A. Gasteiger; "Current Challenges in Catalyst Development for PEM Water Electrolyzers"; Chemie Ingenieur Technik, Volume 92, 2019-11-20