The insulator unraveled
Scientists have uncovered the detailed structure of the aluminum oxide surface, a challenge that has baffled researchers for decades
Advertisement
Aluminum oxide (Al2O3), also known as alumina, corundum, sapphire, or ruby, is one of the best insulators used in a wide range of applications: in electronic components, as a support material for catalysts, or as a chemically resistant ceramic, to name a few. Knowledge of the precise arrangement of the surface atoms is key to understanding how chemical reactions occur on this material, such as those in catalytic processes. Atoms inside the material follow a fixed arrangement, giving rise to the characteristic shapes of crystals. On the surface, however, the structure deviates from that inside the crystal. The strongly insulating nature of alumina hindered experimental studies, and the surface structure evaded precise determination for more than half a century. Researchers at TU Wien and the University of Vienna have now solved the complex structure of the Al2O3 surface, a puzzle listed in 1997 as one of the “Three mysteries of surface science”. The research group led by Jan Balajka and Ulrike Diebold recently published their findings in the journal Science.
High-resolution microscopy identifies surface atoms
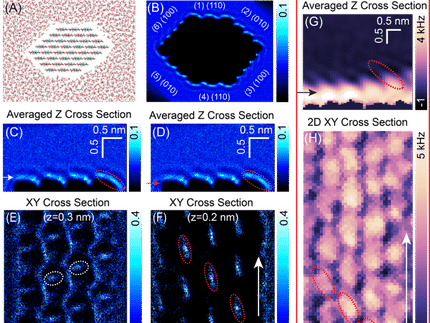
The research team used noncontact atomic force microscopy (ncAFM) to analyze the surface structure. This method generates images of the surface structure by scanning a sharp tip mounted on a quartz tuning fork at a close distance from the surface. The frequency of the tuning fork changes as the tip interacts with the atoms on the surface without touching the material. Johanna Hütner, who performed the experiments, explains: "In an ncAFM image, one can see the location of atoms, but not their chemical identity. We overcame the lack of chemical sensitivity by precisely controlling the tip. Attaching a single oxygen atom to the tip apex allowed us to distinguish between oxygen and aluminum atoms on the surface. The oxygen atom on the tip is repelled from other oxygen atoms at the surface and attracted to aluminum atoms of the Al2O3 surface. Mapping the local repulsion or attraction enabled us to visualize the chemical identity of each surface atom directly."
Restructuring stabilizes the surface without changing its composition
The researchers found that the surface rearranges to allow the aluminum atoms at the surface to penetrate into the material and form chemical bonds with the oxygen atoms in the deeper layers. This rearrangement of the first two atomic layers significantly reduces the energy, effectively stabilizing the structure. In contrast to previous beliefs, the numerical ratio of aluminum to oxygen atoms remains unchanged.
The three-dimensional model of the aluminum oxide surface was optimized with machine learning methods. The main challenge was to match the restructured surface with the underlying crystal. "The structure is very complex, resulting in a vast number of possibilities for how the experimentally inaccessible atoms below the surface could be arranged. The state-of-the-art machine learning algorithms combined with conventional computational methods allowed us to examine numerous possibilities and create the stable three-dimensional model of the aluminum oxide surface," states Andrea Conti, who carried out the computational modeling.
"Through the collaborative effort of experimental and computational research, we not only tackled a long-standing mystery by determining the detailed structure of this enigmatic insulator, but also discovered structure design principles applicable to an entire class of materials. Our results pave the way for advancements in catalysis, material science, and other fields," says Jan Balajka, who led the research.
Parts of the experimental setup housing the noncontact atomic force microscope have been patented.