In search of “holy grails” in catalysis
Assessing the recent progress using single-atom catalysts for five “holy grail” reactions
Advertisement
In the field of catalysis, the term "holy grail" reactions refer to those reactions that hold significant scientific, economic, and environmental sustainability value for the future of humanity. These reactions harness abundant and readily available resources on Earth, such as methane (CH4), water (H2O), carbon dioxide (CO2), nitrogen (N2), to produce various valuable chemical products. Despite their significance, these reactions often suffer from low conversion rates and poor selectivity due to the chemical inertness of reactants and the relatively high reactivity of the products. Developing new catalysts to lower the activation energy barrier remains a grand challenge.
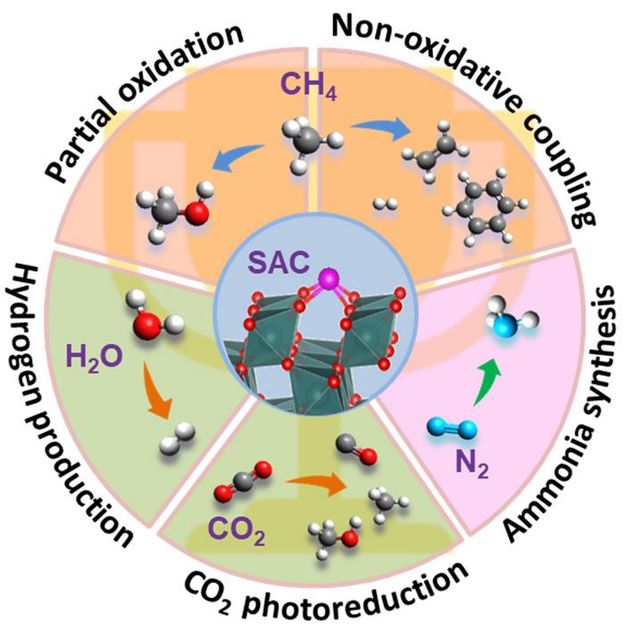
Researchers from National University of Singapore and Dalian Institute of Chemical Physics, CAS, published a perspective assessing the recent advances using structurally well-defined single-atom catalysts (SACs) for several “holy grail” reactions, including oxidative & non-oxidative methane conversion, artificial photosynthesis, and nitrogen activation. Promising progress and the remaining challenges are discussed in using SACs for these invaluable reactions across thermal, electrical, and light-driven catalysis. The significant potential of future development of SACs is also highlighted.
Chinese Journal of Catalysis
Single-atom catalysts (SACs), which contain partially charged single metal atoms with well-defined and tunable structures, represent a promising class of heterogeneous catalysts. Developing novel SACs can not only improve atomic utilization efficiency in active metals, but also promote a deeper understanding of reaction mechanisms and structure-activity relationships. In recent years, emerging SACs have been adopted in those challenging “holy grail” reactions, aiming to improve conversation and selectivity and/or enable milder reaction conditions.
In this context, Prof. Ning Yan (National University of Singapore), Prof. Tao Zhang (Dalian Institute of Chemical Physics, CAS) and co-workers published a perspective that evaluates the latest applications of SACs in five "holy grail" reactions: partial methane oxidation to methanol, non-oxidative methane coupling, photocatalytic water splitting, photocatalytic CO2 reduction, and nitrogen reduction. SACs with structurally well-defined single-atom metal sites possess special geometric and electronic structures, which interacts with inert molecules and regulate the conversion process precisely, achieving selective production of the aim product. Some SACs are composed of molecular-level defined support and unified coordination environments, acting as ideal model catalysts for in-depth mechanistic studies when combined with advanced spectroscopic techniques and DFT calculation. Meanwhile, new catalytic materials like multi-site catalysts, containing two single-atom sites with different coordination structures, or one single-atom site and other non-single-atom sites, can facilitate activation of multiple species, inducing synergistic promotional effects to the reaction.
Potential future directions for the field are outlooked. These breathtaking potential topics include further exploring mechanisms and structure-activity relationships, leveraging advanced information technology for efficient catalyst screening, designing novel catalytic sites to broaden the application scope of catalytic materials, and improving the stability of SACs under the working condition.




























































