Scientists use quantum device to slow down simulated chemical reaction 100 billion times
What happens in femtoseconds in nature can now be observed in milliseconds in the lab
Scientists at the University of Sydney have, for the first time, used a quantum computer to engineer and directly observe a process critical in chemical reactions by slowing it down by a factor of 100 billion times.

Lead authors Vanessa Olaya Agudelo and Dr Christophe Valahu in front of the quantum computer in the Sydney Nanoscience Hub used in the experiment.
Stefanie Zingsheim/University of Sydney
Joint lead researcher and PhD student, Vanessa Olaya Agudelo, said: “It is by understanding these basic processes inside and between molecules that we can open up a new world of possibilities in materials science, drug design, or solar energy harvesting.
“It could also help improve other processes that rely on molecules interacting with light, such as how smog is created or how the ozone layer is damaged.”
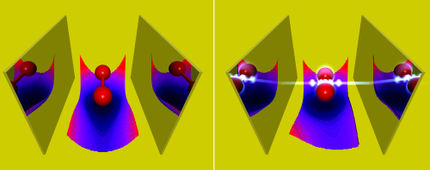
Specifically, the research team witnessed the interference pattern of a single atom caused by a common geometric structure in chemistry called a ‘conical intersection’.
Conical intersections are known throughout chemistry and are vital to rapid photo-chemical processes such as light harvesting in human vision or photosynthesis.
Chemists have tried to directly observe such geometric processes in chemical dynamics since the 1950s, but it is not feasible to observe them directly given the extremely rapid timescales involved.
To get around this problem, quantum researchers in the School of Physics and the School of Chemistry created an experiment using a trapped-ion quantum computer in a completely new way. This allowed them to design and map this very complicated problem onto a relatively small quantum device – and then slow the process down by a factor of 100 billion.
Their research findings are published today in Nature Chemistry.
“In nature, the whole process is over within femtoseconds,” said Ms Olaya Agudelo from the School of Chemistry. “That’s a billionth of a millionth – or one quadrillionth – of a second.”
“Using our quantum computer, we built a system that allowed us to slow down the chemical dynamics from femtoseconds to milliseconds. This allowed us to make meaningful observations and measurements.
“This has never been done before.”
Joint lead author Dr Christophe Valahu from the School of Physics said: “Until now, we have been unable to directly observe the dynamics of ‘geometric phase’; it happens too fast to probe experimentally.
“Using quantum technologies, we have addressed this problem.”
Dr Valahu said it is akin to simulating the air patterns around a plane wing in a wind tunnel.
“Our experiment wasn’t a digital approximation of the process – this was a direct analogue observation of the quantum dynamics unfolding at a speed we could observe,” he said.
In photo-chemical reactions such as photosynthesis, by which plants get their energy from the Sun, molecules transfer energy at lightning speed, forming areas of exchange known as conical intersections.
This study slowed down the dynamics in the quantum computer and revealed the tell-tale hallmarks predicted – but never before seen – associated with conical intersections in photochemistry.
Co-author and research team leader, Associate Professor Ivan Kassal from the School of Chemistry and the University of Sydney Nano Institute, said: “This exciting result will help us better understand ultrafast dynamics – how molecules change at the fastest timescales.
“It is tremendous that at the University of Sydney we have access to the country’s best programmable quantum computer to conduct these experiments.”
The quantum computer used to conduct the experiment is in the Quantum Control Laboratory of Professor Michael Biercuk, the founder of quantum startup, Q-CTRL. The experimental effort was led by Dr Ting Rei Tan.
Dr Tan, a co-author of the study, said: “This is a fantastic collaboration between chemistry theorists and experimental quantum physicists. We are using a new approach in physics to tackle a long-standing problem in chemistry.”