Bacteria cause iron to rust
Anaerobic "rusting" of iron by bacteria with current-conducting pili
iron does not only rust through contact with oxygen and water. Certain bacteria can also decompose iron anaerobically, i.e. in the absence of oxygen. The sediment bacterium Geobacter uses electrically conductive protein threads for this purpose, a research team has now discovered. The oxidized iron then further enhances corrosion through positive feedback, the study says.
Bacteria cause iron to rust
(c) Wiley-VCH
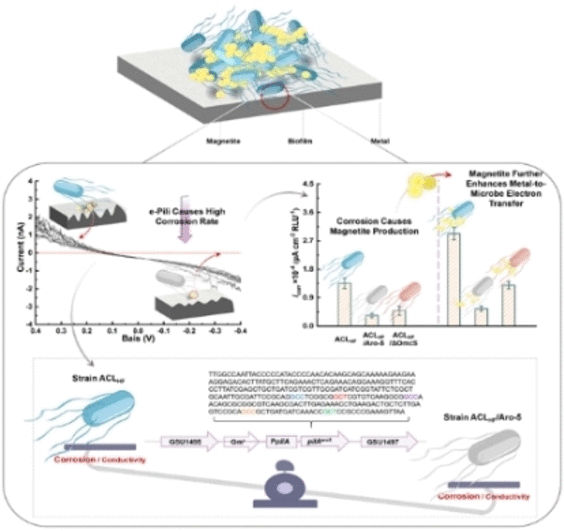
Bacterial biofilms are the cause of so-called microbial-induced corrosion, a dreaded metal corrosion that can destroy entire pipelines. Bacteria such as the anaerobic genus Geobacter, native to river sediments, are among those at work in this type of corrosion. Geobacter does not breathe atmospheric oxygen, but derives energy from pulling electrons from iron, creating magnetite, a natural iron mineral. Exactly how this bacterial iron corrosion works has been unclear.
Dake Xu and his colleagues at Northeastern University in Shenyang, China, have now investigated exactly how microbially induced corrosion occurs in Geobacter. They suspected that protein filaments (pili), which grow from bacteria, play a major role. Geobacter forms e-pili, which are made of conductive proteins and can conduct electricity between bacteria like biological cables. Until now, however, it was not clear whether these E-pili could directly pull electrons from metal surfaces.
To substantiate the suspicion of direct current tapping, the researchers allowed two Geobacter strains to grow into biofilms on a stainless steel surface. One of the two strains formed conductive e-pili, while the other also had pili, but a genetic intervention caused them to consist of less conductive proteins. The researchers found that the E-pili forming bacterial strain was much more comfortable on the steel plate. It grew better and ate deeper holes in the metal. At the same time, it was possible to measure a corrosion current that directly indicates the oxidation of iron.
The team concludes that the bacteria use the e-pili to establish a kind of "current connection" to the metal. E-pili could also provide electrons to bacteria that sit further out in the biofilm and have no direct contact with the metal.
Since magnetite is also formed during the corrosion of iron and this mineral also conducts electricity, the team also investigated its influence on microbial corrosion. It found that when magnetite was added to the biofilm, not only was the growth of Geobacter increased. A stronger corrosion current was also measurable at the metal surface. "The discovery that magnetite, a typical corrosion product, promotes microbially induced corrosion has significant implications for corrosion protection," the researchers believe. They therefore recommend keeping a special eye on their ability to form magnetite for corrosion-free materials.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.




























































