Negatively charged and yet pretty cold
This enables, for example, new investigations of chemical reactions in space
anions, negatively charged ions, are reluctant to be cooled. Physicists led by Matthias Weidemüller from Heidelberg University and Roland Wester from the University of Innsbruck have now developed a method for cooling molecular anions to below 3 Kelvin in a remarkably short time. This enables, for example, new investigations of chemical reactions in space.
Cooling atoms and ions to near absolute zero is routine in many laboratories today. The particles can be very well controlled at these temperatures, and such systems provide an ideal platform for exploring many scientific questions and are the basis for precision clocks or quantum bits. Surprisingly, however, negatively charged ions, known as anions, elude these scientific efforts. They are difficult to cool down. Researchers at Heidelberg University and the University of Innsbruck have now jointly developed a new technique for selectively sorting out the warmest particles from a cloud of molecular ions and thus cooling the remaining molecular ions to below 3 Kelvin.
Evaporative cooling of anions
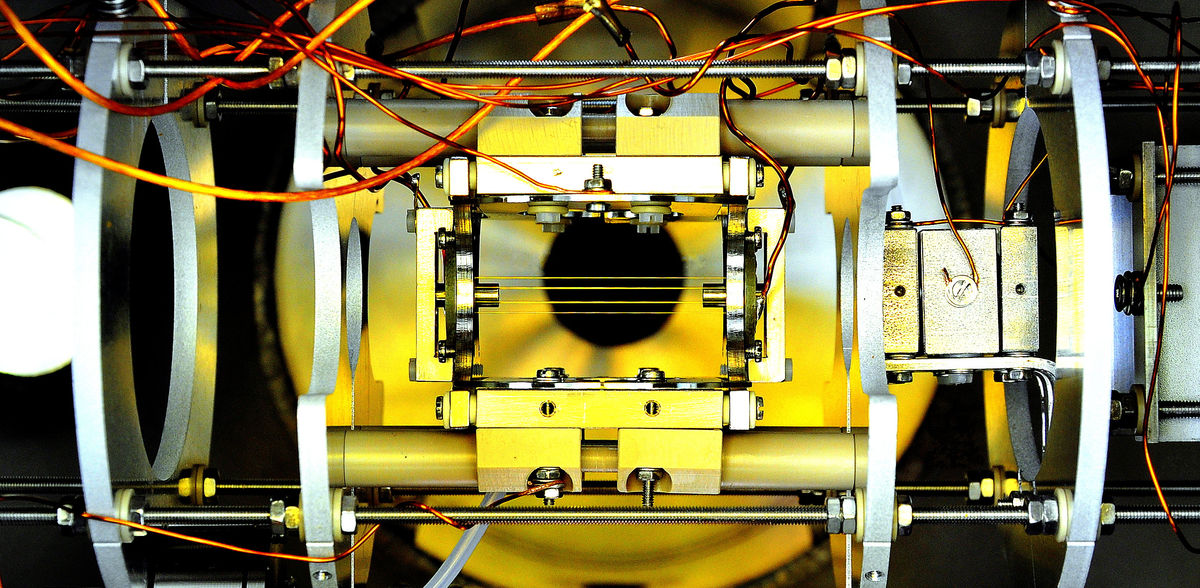
In the experiment, the ions are enclosed in a radio frequency trap and spread along the longitudinal axis of the trap. This allows ions with higher energy to move further away from the center of the trap. "We exploit this to selectively remove these ions from the trap," says Eric Endres of the Department of Ion Physics and Applied Physics. "Using a focused laser beam positioned at the edge of the ion cloud, we neutralize the warmer ions. The photons from the laser thereby detach an electron from the anion, creating a neutral molecule that drops out of the trap." After the higher-energy ions evaporate, the remaining ions cool to a lower temperature. "By slowly moving the laser light towards the trap center, the highest energy anions are evaporated one by one, leading to a temperature of 2.2 Kelvin in less than four seconds," explains Saba Hassan from the Institute for Physics of Heidelberg University.
Previously used techniques allow cooling of anions down to 3 Kelvin. "With our further developed method, this barrier can now in principle be broken for any kind of negatively charged molecule, allowing many new investigations into the fundamentals of nature or, for example, of chemical reactions in space," research group leaders Matthias Weidemüller and Roland Wester are delighted to say.
Original publication
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.