Electron injection builds novel crystalline carbons
Advertisement
Charge injection was widely utilized in tuning the energy level of electrons in semiconductors without altering their microscopic structures. Recently, a research team led by Professor ZHU Yanwu from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) discovered another function of charge injection. By charging C60 molecules periodically arranged in a face center cubic (fcc) lattice with a-Li3N, a novel long-range ordered porous carbon (LOPC) crystal was thereby formed. The result was published in Nature.
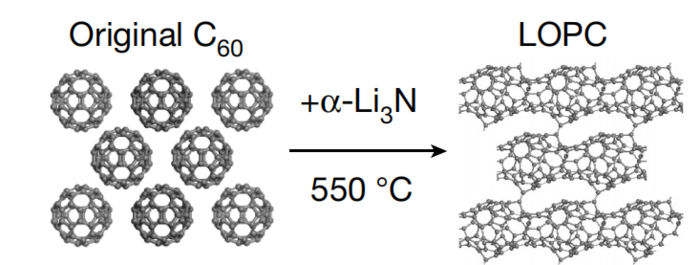
Preparation of LOPC.
Image by PAN Fei et al.
Different from most elemental crystals in which building blocks are multifarious atoms, the building block for LOPC in this study is C60 molecule, commonly known as Soccerene or Buckyball. LOPC possesses the characteristics of both long-range order in the three-dimensional (3D) crystal and partially broken C60 molecules connected as the building blocks.
At elevated temperatures and ambient pressure, a-Li3N would donate electrons to C60 molecules, which will cause the expansion of electron cloud surrounding C60. With the help of dipoles formed, electron cloud at adjacent C60 molecules in the fcc lattice may overlap, forming covalent bonds (C-C bonds) between C60 molecules.
The method of chemical activation using potassium hydroxide, which succeeded in reconstructing graphene to a 3D carbon, has also been adopted by the research team. However, such a 3D carbon owns a disordered structure as the chemical activation is too violent compared with the method of electron injection from α-Li3N. The superiority of electron injection has been demonstrated in preserving the periodic stacking of the nanomaterials that serve as the building blocks.
Many potential applications of LOPC are to be explored in the future. For example, due to the high porosity, it could provide abundant liquid or gas diffusion paths, making it a great candidate in loading of catalysts.
The method of electron injection proposed in the study offers a new approach for constructing new materials in the way of LEGO, allowing the precise control of interface in the crystal structures.