Key to chemical industries’ sustainable future?
The world’s first industrial model of a flow photo-on-demand synthesis system
Advertisement
Patents for this system were filed in Japan in February 2021 and internationally in January 2022. Following the patent announcement in August 2022, the related academic paper was published online in Organic Process Research & Development (OPR&D) on November 11, 2022.
Main Points
- From the common organic solvent chloroform and commercially available alcohol, the researchers successfully synthesized pharmaceutical intermediates and polymers at a highly efficient rate (over 96%) and in a short amount of time (a minute or less of light exposure).
- They showed that continuous production is possible, which cannot be done with conventional batch systems.
- In 2 hours, they successfully synthesized up to ten grams of chemical products (and this can be scaled up)
- They synthesized 10 types of functional carbonates and 3 types of polycarbonates as examples.
- Improved safety compared to the standard method of producing phosgene (a strong exothermic reaction of carbon monoxide and chlorine gas that uses a carbon catalyst). The chloroform used as a precursor in the new method is easy to store safely and the chemical reaction can be controlled by exposure to light.
- The byproduct of this new method is mostly hydrogen chloride (neutralized by alkali), therefore dirt does not build up inside the system apparatus. The reduced need to clean the inside lessens the environmental impact and lowers costs.
- The system achieves continuous production without the additional use of organic solvents.
- This new chemical reaction system is expected to make a significant contribution in the move towards carbon neutral and sustainable societies.
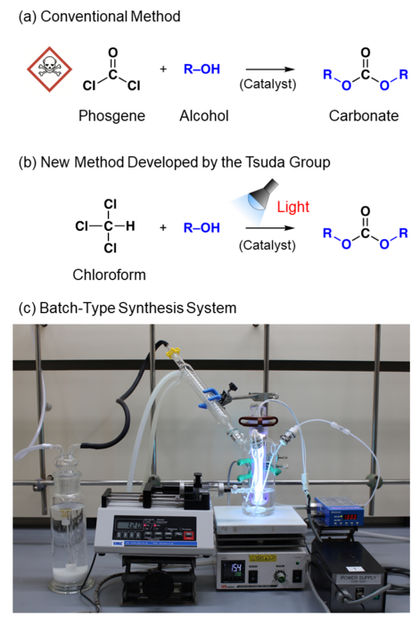
(a) A conventional reaction with phosgene, (b) the photo-on-demand phosgenation reaction developed at Kobe Univ., and (c) a batch-type reaction system for the photo-on-demand chemical synthesis developed at Kobe Univ.
Akihiko Tsuda
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.



























































