Fine-tuned hydrocarbon separation at low energy cost
Metal organic framework membranes that selectively separate hydrocarbons set the stage for more sustainable petrochemical processes
An electrochemical approach developed at KAUST produces molecular-sieving membranes that could enable a cheap energy-efficient separation of light hydrocarbons, such as olefins and paraffins. This separation, critical for the petrochemical industry, usually relies on extremely energy-intensive processes, such as distillation.
KAUST engineers have devised a cheap and more energy efficient method for separating light hydrocarbons, a process that typically consumes a vast amount of energy.
© 2021 KAUST; Sheng Zhou, Osama Shekhah
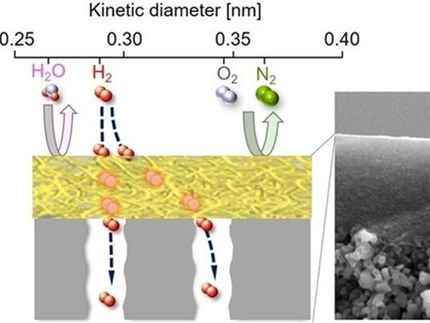
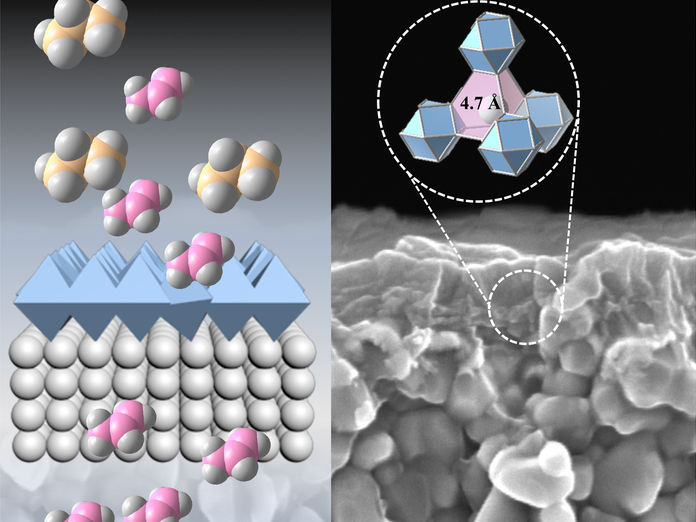
Membrane-based strategies have emerged as promising alternatives to heat-driven separation approaches. Existing polymer-based membranes are not selective enough for hydrocarbon separation and also undergo plasticization, a structural change that alters their pores, at high pressure. On the other hand, highly porous crystalline materials, called face-centered cubic metal-organic frameworks (MOFs), exhibit modular pores with precisely tunable triangular apertures. This means they can be modified to separate light hydrocarbon mixtures. Moreover, the selected MOFs are impervious to plasticization.
Conventional MOF manufacturing methods, such as solvothermal synthesis, involve reacting organic linkers of appropriate length and bulk with a metal source to yield an extended crystalline solid with desired pore geometry. However, these methods require relatively high temperatures, high pressures and long reaction times, which are not easy to scale up.
“Our approach enables the synthesis of continuous MOF films at room temperature and ambient pressure in a short time,” says Mohamed Eddaoudi, who led the study.
The researchers chose preassembled rare-earth and zirconium hexanuclear clusters as building blocks, which reacted with linkers capable of deprotonation on demand to form tiny interconnected crystallites with exposed triangular windows.
Instead of using a base, the electrochemical approach uses external electrons to deprotonate the linkers, which makes it adaptable and easier to control by adjusting the current density and linker concentration. “The protonated linkers are first added and then deprotonated in a controlled manner for immediate consumption, affording the desired crystalline materials,” says Ph.D. student Sheng Zhou.
The resulting nanoporous membranes selectively separated propylene/propane and butane/isobutane mixtures. They also performed well under high pressure, high humidity and highly corrosive conditions similar to industrial hard streams.
The team, explained senior research scientist Osama Shekhah, chose their MOF platform for its proven chemical stability. “If we translate this MOF series into continuous membranes, it will fully meet the industrial requirements and desired robustness under practical industrial extreme conditions,” he adds. “The remaining challenge is to scale up these membranes to the requisite industrial scale.”
The team collaborated with KAUST catalysis scientists to perform simulations that suggested that the zirconium-based membranes can substantially save energy and utility costs if implemented in hybrid membrane–distillation systems producing high-purity propylene.
The team is now trying to scale up the manufacturing process to generate more compact membrane modules and assess their performance under industrial conditions.
Original publication
Other news from the department science
These products might interest you
Anopore™ by Cytiva
Precise filtration made easy with Anopore inorganic membranes
The aluminum oxide filter membrane that can increase the purity or yield of your analyte

Hahnemühle LifeScience Catalogue Industry & Laboratory by Hahnemühle
Wide variety of Filter Papers for all Laboratory and Industrial Applications
Filtration Solutions in the Life Sciences, Chemical and Pharmaceutical Sectors

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.