New procedure will reduce the need for rare metals in chemical synthesis
Researchers are working to improve the long-term sustainability of pharmaceutical and other chemical syntheses
Pharmaceuticals, plastics, and many other chemical products have transformed human life. To prepare these products, chemists often use a catalyst--frequently based on rare metals--at various points in their syntheses. Although rare-metal catalysts are incredibly useful, their limited supply means that their use is unsustainable in the long term. Synthetic chemists need an alternative.
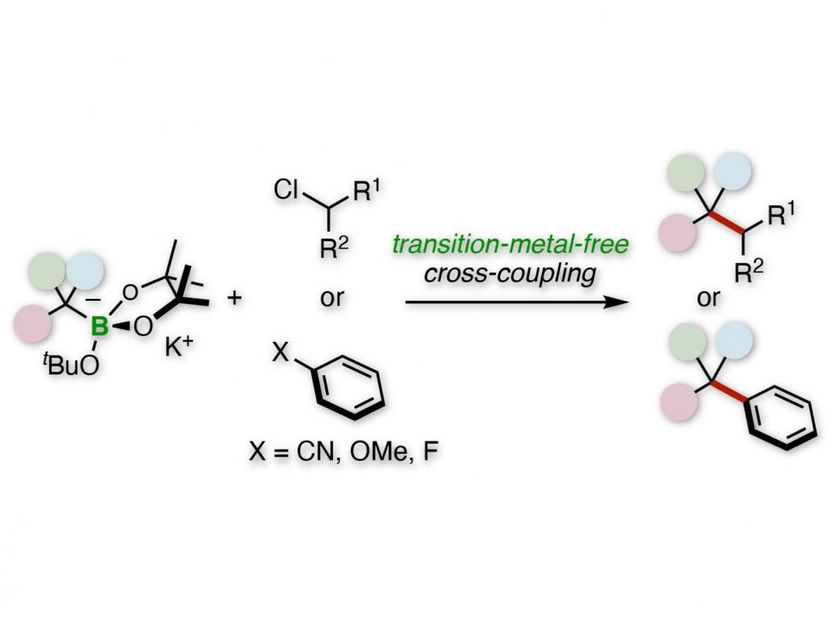
Tertiary alkylative cross-coupling of alkyl or aryl electrophiles.
Kanazawa University
In a study recently published in Angewandte Chemie, researchers from Kanazawa University report such an alternative. Their research on a broad class of chemical reactions that are common in pharmaceutical and other syntheses will pave the way to a more sustainable chemical industry.
The 2010 Nobel Prize in Chemistry went to researchers who used catalysts based on palladium metal to perform a common type of chemical reaction known as cross-coupling. Such catalysts work very well for synthesizing what are known as congested quaternary carbon centers, which are common in molecules used in agriculture and medicine. However, for long-term sustainability, researchers need an alternative to rare-metal catalysts.
"We used benzylic organoborates to perform tertiary alkylative cross-coupling of aryl or alkyl electrophiles," says Hirohisa Ohmiya, corresponding author of the study. "Our procedure does not use rare elements and is a straightforward route to quaternary carbon centers."
The researchers' initial studies consisted of a tertiary benzylboronate that is first activated by a potassium alkoxide base to become a benzyl anion. This anion then undergoes a cross-coupling reaction with a secondary alkyl chloride electrophile.
"The reaction has broad scope," explains corresponding author Hirohisa Ohmiya. "For example, replacing the phenyl group of the boronate with various aromatic rings was successful, and the electrophile can be a wide range of rings and linear chains."
Subsequent studies replaced the secondary alkyl chloride with various aryl nitriles, aryl ethers, and aryl fluorides. Many of these reactions were successful, such as those with 4-cyanopyridine and 4-fluorophenylbenzene.
A comment in Nature on November 19 indicates that the COVID-19 pandemic has disrupted supply chains to various rare metals that are pertinent to the chemical industry. Hundreds of mines and factories have been closed, and many national borders are more restricted than before the pandemic. A long-term solution to supply chain disruptions is to develop synthetic protocols that don't use rare metals. The research described here is an important part of that effort and will help make chemical syntheses more sustainable for future generations.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.




























































