Researchers develop method to improve skeleton of common chemicals
Every chemical, from the simplest to the most complex, have a structural skeleton of atoms. The atoms can be added or removed to transform the chemical into different types, for use in different applications. For many pharmaceutical and agricultural chemicals, the skeleton consists of a multi-membered carbon ring called a carbocycle.
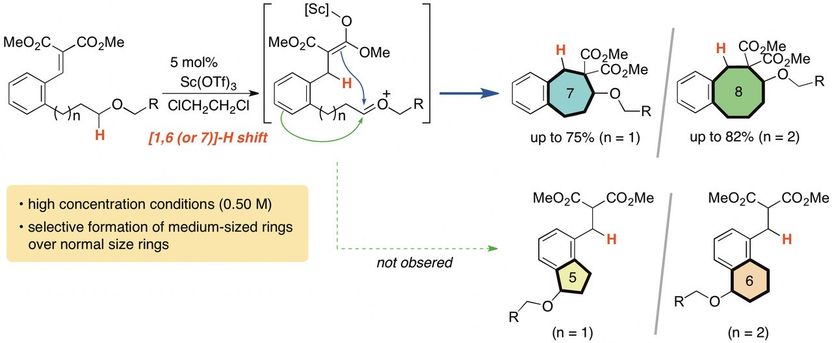
Researchers in Japan developed a new method to produce more complicated and medium-sized (seven- and eight-membered) carbocycles.
Keiji Mori, TUAT
It's incredibly difficult to make a carbocycle with more than five or six members, but a research team at Tokyo University of Agriculture and Technology (TUAT) in Japan has developed a new method that can easily produce seven- and eight-membered carbocycles.
"Generally speaking, formation of middle-sized rings is a difficult issue because of their instability and disorder," said Keiji Mori, associate professor in the Department of Applied Chemistry in the Graduate School of Engineering at TUAT. "In this study, we developed a simple and effective method for the construction of seven- and eight-membered carbocycles."
Mori and co-author Yuna Otawa, also with the Department of Applied Chemistry in the Graduate School of Engineering at TUAT, used a process called the "internal redox process."
The carbocycles include carbon atoms bonded to hydrogen atoms. The system of carbocycles is oxidized, which involves the chemicals rearranging and exchanging components. This weakens the bonds between carbons and hydrogens, allowing hydrogen atoms to bond to a different carbon. The researchers induced this process in a cyclical manner, causing hydrogen shift to distal carbon, leading to a cleanly arranged, medium-sized (seven- and eight-membered) carbocycles. Noteworthy point is that their formation is overwhelmingly favored compared to five- or six-membered ring formation, which are more facile process.
"The construction of seven-membered or larger carbocycles is a major research topic in modern synthetic organic chemistry," Mori said. "Many organic chemists have devoted much time and effort to the development of an effective method for the synthesis of this class of skeleton, but most of the reported methods require relatively dilute conditions and special precautions to suppress unwanted intermolecular reactions, thereby decreasing the practicality of the synthesis."
According to Mori, the new method can be performed without special precautions and without undesired molecular effects.
"Our ultimate goal is the development of a synthetic method to two types of medium-sized rings fused-polycycles, which is difficult to achieve with current conventional methods," Mori said.
Original publication
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Room temperature superconductivity - One step closer to the Holy Grail of physics
Borealis to strengthen its innovation capability
Sika expands its concrete admixtures business with partnership in Western China
Seward moves to larger premises to double production capabilities
BASF increases prices for polymer dispersions, polymer powders and acrylic resins
Bac2 announces blendable latent acid catalysts for energy efficient control of heat activated polymerisation processes
New self-stretching material developed - No limit to number of times material can change shape
Solutia Raises Prices for Saflex(R) and Vanceva(R) PVB Films