Water could modulate the activity and selectivity of CO2 reduction
As an alternative to the depletion of fossil resources, the reduction of CO2 emitted from fossil fuel combustion into valuable chemicals and fuel has drawn increasing attention. Due to the highly thermodynamic stability of CO2, it is still very challenging to find a sustainable and cost-efficient route to selectively convert inert CO2 at a high conversion rate under mild conditions. The water could also be a significant factor governing the reaction mechanism. On the one hand, water is one product accompanied with CH3OH. H2O might trigger the autocatalysis to accelerate CO2 activation and methanol production. On the other hand, Cu is also a highly active catalyst for water-gas shift (WGS) reaction and its reverse reaction (RWGS). The water is also possible to launch or to suppress the side reaction, which could influence the selectivity.
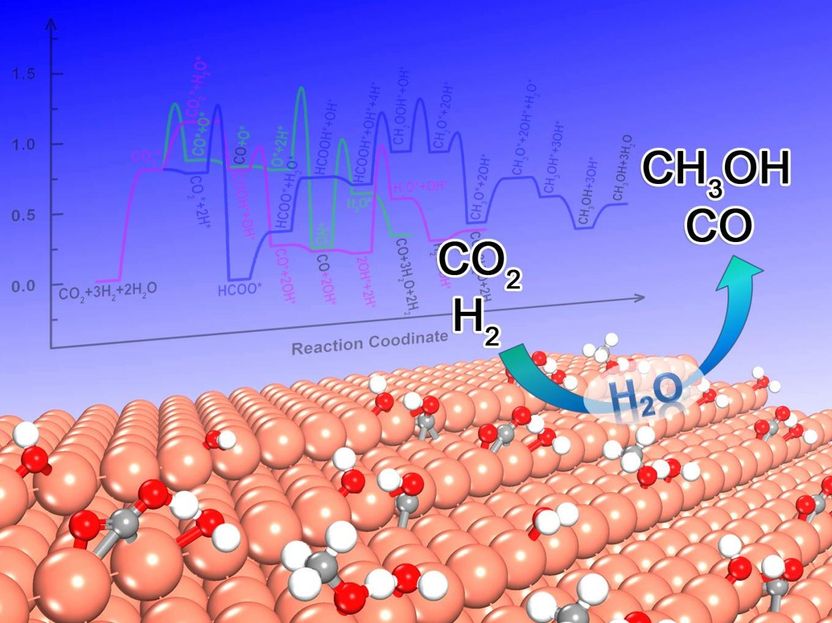
The schematic diagram of water effect on CO2 hydrogenation over stepped Cu(211) surface.
©Science China Press
Very recently, Dr. Cao and Prof. P. Hu's group at East China University of Technology systematically analyzed the effect of surface water on each elementary step to disclose its role in CO2 hydrogenation over the stepped Cu(211) surface. The influence of water on CO2 hydrogenation was respectively discussed at the levels of the electronic structure, energy and kinetics. The mechanisms between in the absence and presence of water were quantitatively compared based on microkinetic simulation upon density functional theory (DFT) calculations.
It has been found that the autocatalysis of water could be in favor of the formation of COOH intermediate through the proton transfer. This accelerates the CO generation while does not improve the methanol synthesis, giving rise to the higher CO2 conversion and the selectivity towards CO. In addition, the microkinetic analysis also sheds light on the fact that the too high initial partial pressure of water will thermodynamically inhibit the CO2 conversion due to the too high coverage of OH*. Hence, it is the first time to disclose how to modulate the activity and selectivity of CO2 hydrogenation through changing the H2O pressure.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.




























































