Uncovering atomic movements in crystal
Scientists can spend a long time in heated debates over tiny details – for example, how and whether atoms in a crystal move when heated, thereby altering the symmetry. Using computer simulations for the mineral lead telluride on the CSCS supercomputer “Piz Daint”, ETH researchers have resolved a long-standing controversy.
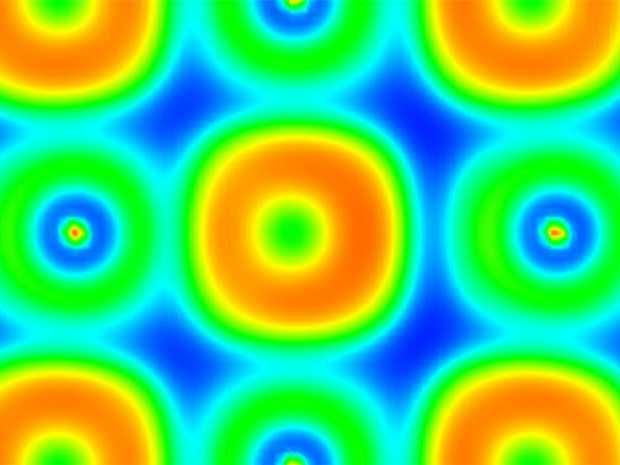
Calculated correlated local dipoles in PbTe. The colors illustrate the asymmetric electrons around the Pb and Te atoms which generate the dipoles.
Institute of Materials Theory/ETH Zurich
To outsiders, scientific questions can sometimes look like hair-splitting. These questions, however, are often crucial, as in materials science: the commercial use of a material stands or falls on its properties. One question that very much depends on such hair-splitting is the one that Boris Sangiorgio pursued in his doctoral thesis. In ETH Professor Nicola Spaldin’s research group at the Institute of Materials Theory, Sangiorgio used the supercomputer “Piz Daint” to examine how lead telluride (PbTe) behaves when it warms up. Lead telluride appears in nature as altaite, a sulfosalt mineral. Altaite can convert heat energy into electrical energy, meaning it has thermoelectric properties.
Mars rover moves using lead telluride
Thermoelectric materials became popular in aerospace in the 1960s, and remain in widespread use today; for example, a thermoelectric generator made of lead telluride powers the Mars rover Curiosity since 2012.
About seven years ago, however, a study on lead telluride ignited a dispute among materials scientists. Back then, researchers came to the conclusion that when lead telluride is heated, the phenomenon of emphanisis occurs. In simple terms, heating causes the lead atoms to shift locally in the crystal, reducing local symmetry. Previously, only the reverse process had been known: heating caused an increase in symmetry.
Emphanisis remained poorly understood until Spaldin’s team examined this phenomenon in lead telluride using the supercomputer. The simulations show that when the mineral is heated, the symmetry is broken locally. However, when the crystal is viewed as a whole, the original cubic symmetry is retained.
For real experiments with the mineral, the scientists worked in collaboration with researchers at the X-ray platform in the Department of Materials at ETH and used an X-ray scattering technique that provided high-precision visibility of the atomic crystal structure. The results from these trials corresponded very closely with those of the simulation, thereby confirming the simulation results. The researchers were then able to go a step further in the simulations than in the experiment and discover what was behind the process of emphanisis in lead telluride.
New phenomenon
The simulations show that heating the crystal leads to strong acoustic and optical vibrations. These vibrations overlap and are coupled together, which produces a phenomenon that has never been observed before: due to the coupled vibrations, correlated dipoles form in the crystal, with pairs of Pb and Te atoms both fluctuating and orienting according to their charges. “Seen as a whole, however, the atoms are still in a highly symmetrical position,” says Sangiorgio. The global symmetry is retained. The researchers suspect that this process is essential for lead telluride’s thermoelectric behaviour. It could also be true of other materials (so called ferroelectrics) that, like lead telluride, are close to a ferroelectric phase transition.
Versatile use of thermoelectric materials
“The functionality of lead telluride is likely based on a sensitive balance between electronic and structural properties,” says Sangiorgio. Understanding the local structure and dynamics of lead telluride is essential for scientists to explain the behaviour of the material. These findings will help them to create or find more efficient thermoelectric materials in future. Thermoelectric materials are not only of interest in aerospace research; they could also help to make more efficient use of waste heat from incineration plants or cars in electricity generation.