Lighting the way for more efficient LEDs
Researchers at the U.S. Naval Research Laboratory (NRL) Center for Computational Materials Science, working with an international team of physicists, have revealed that nanocrystals made of cesium lead halide perovskites (CsPbX3), is the first discovered material which the ground exciton state is "bright," making it an attractive candidate for more efficient solid-state lasers and light emitting diodes (LEDs).
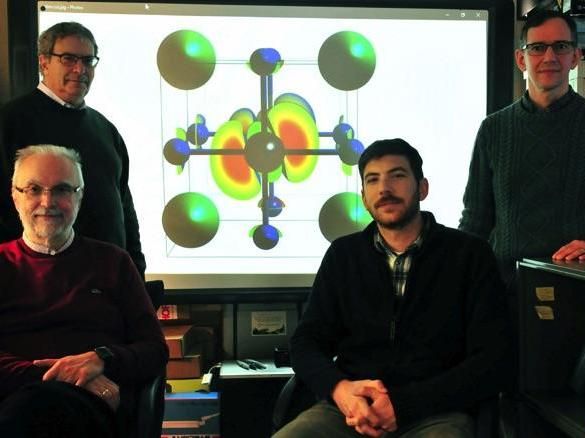
Researchers at the US Naval Research Laboratory Center for Computational Materials Science, working with an international team of physicists, show that cesium lead halide perovskites nanocrystals (CsPbX3) -- which atomic structure is shown on rear screen -- emit light much faster than conventional light emitting materials. This property is enabling more efficient lasers and LEDs with larger emission power at lower energy use, as well as faster switching for communication and sensors. Pictured standing (l-r) are NRL researchers Dr. Alex Efros and Dr. Noam Bernstein; sitting (l-r) are Dr. John Michopoulos and Dr. John Lyons.
US Naval Research Laboratory/James Marshall
"The discovery of such material, and understanding of the nature of the existence of the ground bright exciton, open the way for the discovery of other semiconductor structures with bright ground excitons," said Dr. Alexander Efros, research physicist, NRL. "An optically active bright exciton in this material emits light much faster than in conventional light emitting materials and enables larger power, lower energy use, and faster switching for communication and sensors."
The work, which was partially sponsored by the Office of Naval Research through a program managed by Dr. Chagaan Baatar, studied lead halide perovskites with three different compositions, including chlorine, bromine, and iodine. Nanocrystals made of these compounds and their alloys can be tuned to emit light at wavelengths that span the entire visible range, while retaining the fast light emission that gives them their superior performance.
Semiconductors emit light when bound pairs of electrons and holes, known as excitons, recombine in a process called radiative decay.
"In all known semiconductors and semiconductor nanostructures, the lowest energy state for a bound electron-hole pair is a 'dark' state," said Efros. "This means the material emits light slowly and weakly."
Because in perovskite nanocrystals the lowest energy exciton is bright, the time it takes for the electron and hole to recombine and emit light, known as their radiative lifetime, is 20 times faster than conventional materials at room temperature and 1000 times faster at cryogenic temperatures.
It is known, that quantum-dot based LEDs, or QLEDs, can suffer from "droop," or reduced efficiency, at high pumping intensity due to processes that dissipate the energy of excitons before they have time to emit light. The decreased radiative lifetime should make it possible for LEDs based on these perovskites to use all of the energy input to create light before it is dissipated through slower processes.
"The increased rate of light emission of these materials holds great promise for various technological applications that rely on LEDs and lasers," Efros said. "In principle, the 20 times shorter lifetime could therefore lead to 20 times more intense LEDs and lasers." The power of a laser depends on the gain of the material it is made of, and this gain is proportional to the radiative emission rate.
Communication in free space using visible light, which makes it possible to transmit information in tight beams for long distances without fiber optic or copper cables, would also benefit from the increased light emission rates. "The maximum bandwidth of the communication system is limited by the rate at which the LEDs can turn on and off, and the shorter radiative lifetime translates directly into faster switching and therefore a higher data transmission rate," says Efros.
Original publication
Michael A. Becker, Roman Vaxenburg, Georgian Nedelcu, Peter C. Sercel, Andrew Shabaev, Michael J. Mehl, John G. Michopoulos, Samuel G. Lambrakos, Noam Bernstein, John L. Lyons, Thilo Stöferle, Rainer F. Mahrt, Maksym V. Kovalenko, David J. Norris, Gabriele Rainò & Alexander L. Efros; "Bright triplet excitons in caesium lead halide perovskites"; Nature; 2018
Original publication
Michael A. Becker, Roman Vaxenburg, Georgian Nedelcu, Peter C. Sercel, Andrew Shabaev, Michael J. Mehl, John G. Michopoulos, Samuel G. Lambrakos, Noam Bernstein, John L. Lyons, Thilo Stöferle, Rainer F. Mahrt, Maksym V. Kovalenko, David J. Norris, Gabriele Rainò & Alexander L. Efros; "Bright triplet excitons in caesium lead halide perovskites"; Nature; 2018
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.