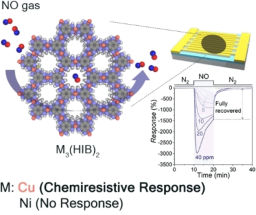
The uncalculability of electron systems
Theoretical physicists of the Max Planck Institute of Quantum Optics reveal limitations of Density Functional Theory using Quantum Information Theory tools
The electric and magnetic properties of solids are impossible to calculate exactly: The complex interactions of the many electrons which underly these phenomena cannot be computed even by the most powerful classical computers. Here, the central task is to determine the ground state of the electrons moving in the field of the positively charged nuclei. The most widely used method for treating such systems is density Functional Theory, which reduces the many-body problem to a single particle interaction. As Dr. Norbert Schuch, scientist in the theory division of Prof. Ignacio Cirac at the Max Planck Institute of Quantum optics in Garching, and Prof. Frank Verstraete from the University of Vienna, report in Nature Physics, there exist however fundamental limitations to the applicability of this theory. The scientists succeeded by using methods developed in Quantum Information Theory, demonstrating that these methods can give deep insights beyond the development of quantum computers.
One of the central problems in quantum mechanics is to determine the ground state of a complex system consisting of many interacting electrons. An example taken from chemistry is the geometry of large molecules: the spatial arrangement of the atoms in the molecule is the one for which the energy of the electrons moving in the field of the nuclei is minimized. Thus, by determining the ground state of the electrons one can infer the three-dimensional structure of the molecule. The same holds for solids: Their electric and magnetic properties, including exotic phenomena such as high-temperature superconductivity, ultimately originate from the motion of the electrons in the periodic potential of the positively charged nuclei.
Density Functional Theory (DFT) makes use of the fact that the complex interaction of the electrons is the same in all these cases and encapsulates it in some kind of "black box", the so-called "universal functional". By using this functional, every many-electron problem can in principle be rephrased as a single-particle problem which can then be solved relatively easily. The challenge consists in finding this functional, and in practice, often more specific problem-dependent approximations are being used.
In their work, Schuch and Verstraete investigate the limits of the applicability of DFT: Is it possible to find this universal functional which would considerably simplify the treatment of many-electron systems - or are there fundamental bounds which prohibit this? To this end, they use methods of quantum complexity theory, a subarea of quantum information science, which aims at classifying problems according to their difficulty, especially concerning the question whether they can be efficiently solved by quantum computers. Whereas e.g. quantum computers can often simulate the time evolutions of quantum systems efficiently, computing ground states of complex quantum systems poses a hard problem even for a quantum computer.
In their work, Schuch and Verstraete prove on the one hand that ground states of many-electron systems are hard to compute even for quantum computers. On the contrary, they show that these problems can be solved efficiently even by classical computers using Density Functional Theory, given the universal functional is known. This shows that in these cases it is fundamentally impossible to compute the functional and explains the need for more specific approximations. This exhibits that despite its broad applicability, there are fundamental limitations to Density Functional Theory.
Original publication: Norbert Schuch and Frank Verstraete; "Computational Complexity of interacting electrons and fundamental limitations of Density Functional Theory"; Nature Physics, Published online: 23 August 2009
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.



![[Fe]-hydrogenase catalysis visualized using para-hydrogen-enhanced nuclear magnetic resonance spectroscopy](https://img.chemie.de/Portal/News/675fd46b9b54f_sBuG8s4sS.png?tr=w-712,h-534,cm-extract,x-0,y-16:n-xl)