From graphene to graphane, now the possibilities are endless
Ever since graphene was discovered in 2004, this one-atom thick, super strong, carbon-based electrical conductor has been billed as a "wonder material" that some physicists think could one day replace silicon in computer chips.
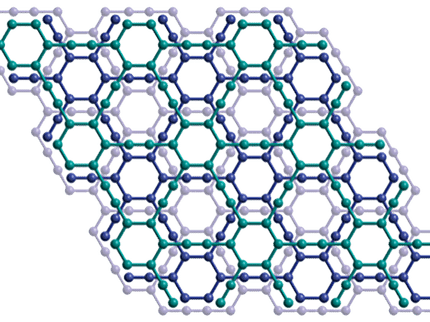
But graphene, which consists of carbon atoms arranged in a honeycomb lattice, has a major drawback when it comes to applications in electronics – it conducts electricity almost too well, making it hard to create graphene-based transistors that are suitable for integrated circuits.
In August's Physics World, Kostya Novoselov - a condensed-matter physicist from the Manchester University group that discovered graphene -- explains how their discovery of graphane, an insulating equivalent of graphene, may prove more versatile still.
Graphane has the same honeycomb structure as graphene, except that it is "spray-painted" with hydrogen atoms that attach themselves to the carbon. The resulting bonds between the hydrogen and carbon atoms effectively tie down the electrons that make graphene so conducting. Yet graphane retains the thinness, super-strength, flexibility and density of its older chemical cousin.
One advantage of graphane is that it could actually become easier to make the tiny strips of graphene needed for electronic circuits. Such structures are currently made rather crudely by taking a sheet of the material and effectively burning away everything except the bit you need. But now such strips could be made by simply coating the whole of a graphene sheet – except for the strip itself - with hydrogen. The narrow bit left free of hydrogen is your conducting graphene strip, surrounded by a much bigger graphane area that electrons cannot go down.
As if this is not enough, the physicists in Manchester have found that by gradually binding hydrogen to graphene they are able to drive the process of transforming a conducting material into an insulating one and watch what happens in between.
Perhaps most importantly of all, the discovery of graphane opens the flood gates to further chemical modifications of graphene. With metallic graphene at one end and insulating graphane at the other, can we fill in the divide between them with, say, graphene-based semiconductors or by, say, substituting hydrogen for fluorine?
As Professor Novoselov writes, "Being able to control the resistivity, optical transmittance and a material's work function would all be important for photonic devices like solar cells and liquid-crystal displays, for example, and altering mechanical properties and surface potential is at the heart of designing composite materials. Chemical modification of graphene – with graphane as its first example – uncovers a whole new dimension of research. The capabilities are practically endless."
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.