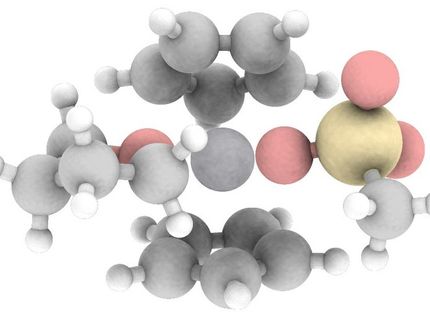
Double whammy for platinum cluster
Scientists in Canada have determined the structure of a carbonatoplatinum(IV) complex that shows an unprecedented geometry
Advertisement
Richard Puddephatt and colleagues from the University of Western Ontario, Canada, oxidised a Pt(II) chelate complex with hydrogen peroxide to form carbonatoplatinum(IV) crystals. The X-ray crystallography of the product showed that the crystals contained two distinct Pt(IV) moieties. Firstly, it shows a mononuclear carbonato complex, which has relevance to anti-cancer pharmaceuticals such as cisplatin. Secondly, the tetranuclear species has a face-bridged double cubane structure, with two vertices missing.
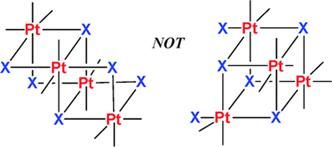
Back in 1907, Pope and Peachy famously reported the first synthesis of a trimethylplatinum halide, which showed to have cubane geometry. All subsequent research into tri- and dimethylplatinum halide compounds continues to support having a cubane structure. However, the organoplatinum(IV) compound reported by Puddephatt and his colleagues has shown that this geometry is not always to be expected, as they observed a double cubane geometry.
‘The discovery was made serendipitously during attempted synthesis of methyl(hydroxo) platinum(IV) compounds as models for intermediates in catalytic oxidation,’ says Puddephatt. ‘The old paradigm for structures of organoplatinum(IV) compounds is therefore broken but the new one needs to be tested.’ The main reason why this has not been solved sooner is because the majority of the organoplatinum(IV) compounds are insoluble and so X-ray structure determination is not always possible. Puddephatt explains that ‘structure determination is always a major challenge, but newer structure determination methods may offer a solution, or new synthetic methods may yield crystalline compounds.’
Either way this work should stimulate further research into determining new (and old) organoplatinum(IV) structures, bringing scientists a step closer to fully understanding platinum chemistry and its geometries.
Original publication: Muhieddine S. Safa et. al., Chem. Commun. 2009.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.