Si-F bond breaking
Advertisement
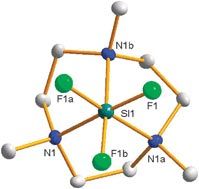
Scientists in the UK have prepared a unique silicon(IV) cation by breaking a very strong Si-F bond under mild conditions. Chemists at the University of Southampton have prepared the trifluorosilicon(IV) cation [SiF3(Me3tacn)]+, by reaction of SiF4 with Me3tacn under anhydrous conditions and compared its electronic and structural properties to tetrafluoro complexes.
Silicon chemistry has major applications in electronics, such as semiconductors, organic chemistry and medicine. The coordination chemistry of silicon has therefore seen renewed interest in order to develop the underlying chemistry further.
Silicon tetrafluoride presents a significant challenge in coordination chemistry due to the Si-F single bond being the strongest bond involving a Group 14 element of the Periodic Table and therefore fluoride is not readily substituted.
Levason and his team have shown that the very strong Si-F bond can be broken by using a neutral amine under mild conditions, which they attribute to the very strong binding displayed by the tacn macrocycle. This provides a strong driving force for tridentate coordination and a readily isolable cation.
‘We are now looking at the chemistry of silicon fluoride and the heavier halides with other neutral donor macrocyclic and open-chain ligands to develop a better understanding of the factors determining the substitution of the halides and the stability of the complexes,’ says Levason.
Original publication: Levason et. al., Chem. Commun. 2009.
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.






























































