Scripps Research scientists watch membrane fission in real time
Researchers at The Scripps Research Institute have solved one of biology's neatest little tricks: they have discovered how a cell's outer membrane pinches a little pouch from itself to bring molecules outside the cell inside — without making holes that leak fluid from either side of the membrane. In Cell, the scientists describe creating a system in which they can watch, in real time under a light microscope, cell membranes bud and then pinch off smaller sack-like "vesicles."
This process is only possible, Scripps Research scientists say, because a single molecule, dynamin, forms a short "collar" of proteins around a bit of the membrane that has emerged from the "parent" membrane, and then squeezes it tight, cleanly separating the new "daughter" vesicle.
"Doing this without leaking is quite a feat," says Sandra Schmid, Ph.D., chair of the Scripps Research Department of Cell Biology, who authored the paper with Thomas Pucadyil, Ph.D., a postdoctoral researcher in her lab. "A cell's outside environment is very nasty, and if any of that toxic fluid got into the cell, it would kill it."
The findings contradict the prevailing notion of how these vesicles are tied off from the membrane, and also suggest that this elegant little action may be ubiquitous throughout the cell, which must form millions of vesicles to move molecules between membrane organelles within the cell.
In this process, called endocytosis, cells take up materials (including hormones, nutrients, antibodies, and fats such as cholesterol) from the bloodstream by engulfing them in inward folds of the cell membrane that then close up, pinch off, and move into the cell as the cargo-laden vesicles.
This study focused on the proteins that actually pinch off the vesicle. The prevailing theory has been that a molecule called dynamin is responsible for the process, but that it does it by first forming a very long chain spiral, shaped like a Slinky® Toy, around the neck of the vesicle. Once in place, it was hypothesized that the dynamin spiral tightened. This constriction requires the presence of GTP, a small molecule that provides the fuel for dynamin, a GTPase, to perform the action. Many researchers believed that the constriction helped to pinched off the vesicle but that dynamin could not function alone.
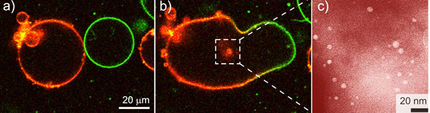
That's not what Schmid and Pucadyil found. To see exactly what happens, Pucadyil, a biophysicist, invented a new membrane template to study the process as it happens. He used tiny glass beads — 200 of them could fit on to the head of a pin — and formed membranes around each one of them. "The beauty of this is that we can see these beads using a normal light microscope," Schmid says. Each bead contains a loosely fitting bi-layer membrane, just like a cell would have.
The scientists then added dynamin alone and watched what occurred. The beads formed long hairy spirals, just as the prevailing hypothesis would predict, but surprisingly no fission (the pinching off of daughter vesicles) took place even when they added GTP later.
The researchers then tried a slightly different condition. They added GTP first and then dynamin to the membrane beads. In this case, "we didn't see any hairy structures at all – just little vesicles popping off the beads," Schmid says. "It was an exciting moment when Thomas first saw the small daughter vesicles emerge from the mother 'ship' and dance around it."
Close analysis demonstrated that only in the constant presence of GTP does dynamin form short collars at the neck of the vesicles that then tighten to break them free of the membrane.
An accompanying study in Cell led by Vadim Frolov and Josh Zimmerberg, which includes Schmid as a co-author, extends these findings into a "unified theory as to how intracellular vesicle formation works," she says. "Dynamin is often involved in forming the vesicles that help move molecules and chemicals inside cells, so this work provides a model as to how these other processes occur."
Schmid says the new technology developed at Scripps Research, which they dub SUPER (fluid supported bi-layers with excess membrane reservoir) templates can now easily be used to test these theories. "It is easy, done in real time, and can be seen through a simple microscope," she says.
Topics
Organizations
Other news from the department science
These products might interest you
Anopore™ by Cytiva
Precise filtration made easy with Anopore inorganic membranes
The aluminum oxide filter membrane that can increase the purity or yield of your analyte

Hahnemühle LifeScience Catalogue Industry & Laboratory by Hahnemühle
Wide variety of Filter Papers for all Laboratory and Industrial Applications
Filtration Solutions in the Life Sciences, Chemical and Pharmaceutical Sectors

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.